Notice
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 : National guideline implementation and national registries.
- document 1 document 2 document 3
- niveau 1 niveau 2 niveau 3
Descriptif
Title : Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 : National guideline implementation and national registries
• NICE
Speaker: Suzanna HARDMAN, London, GBR
• German CHF registry: REFLECT –HF
Speaker: Carsten TSCHOEPE, Berlin, GER
• Eurobservational, the ESC Heart failure Registry
Speaker: Aldo MAGGIONI, Florence, ITA
Abstract : Multidisciplinary expert workshop: achievements, challenges and barriers to implementations of the ESC 2012 chronic heart failure
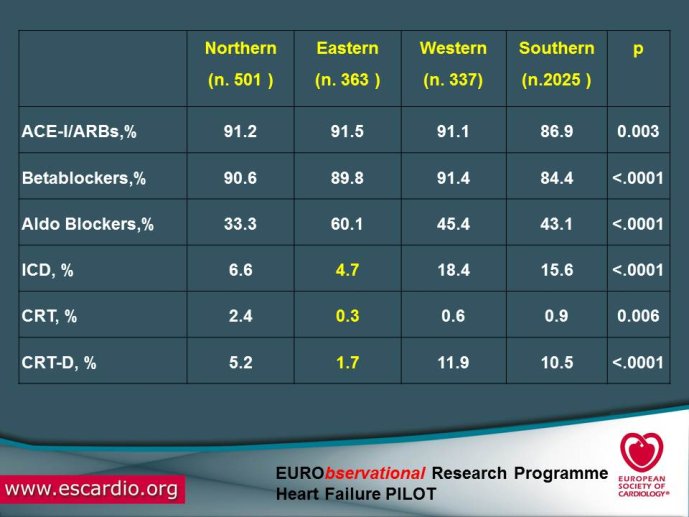
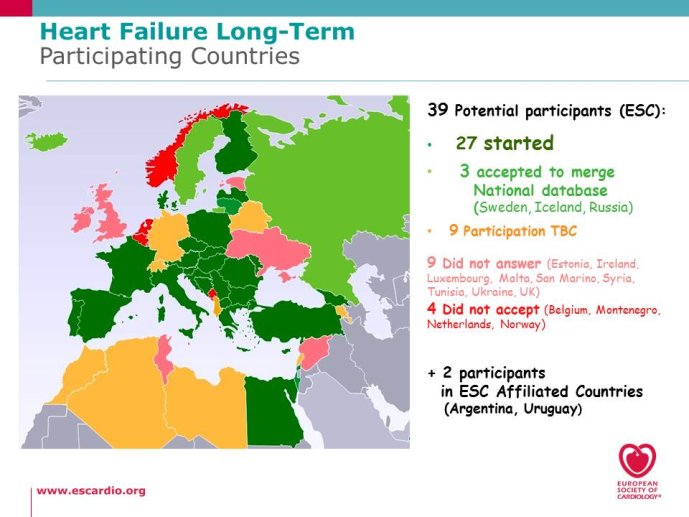
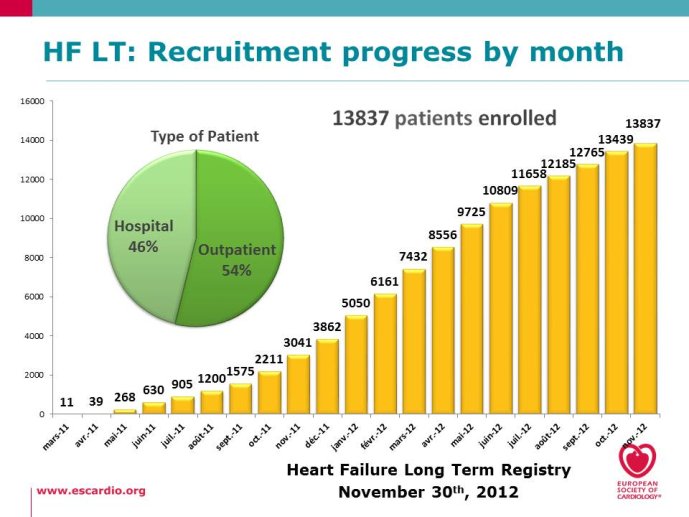
Eurobservational Research Program, the ESC Heart Failure Long Term Registry
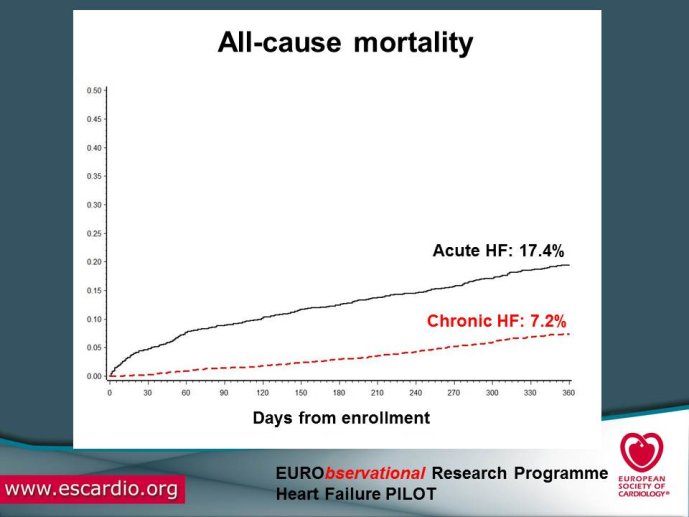
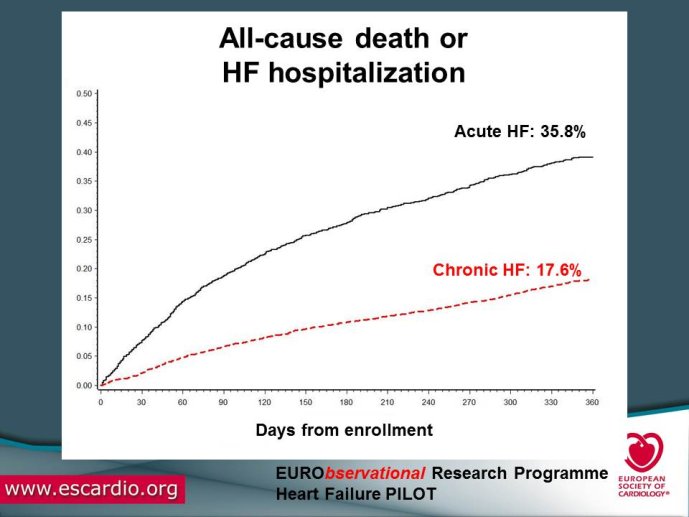
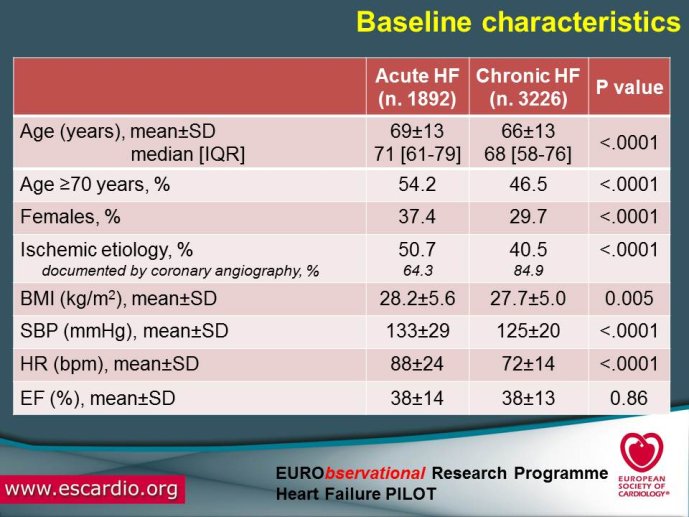
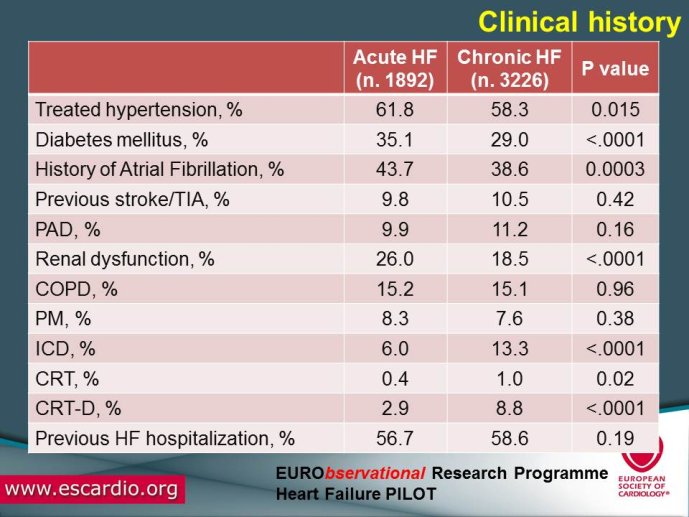
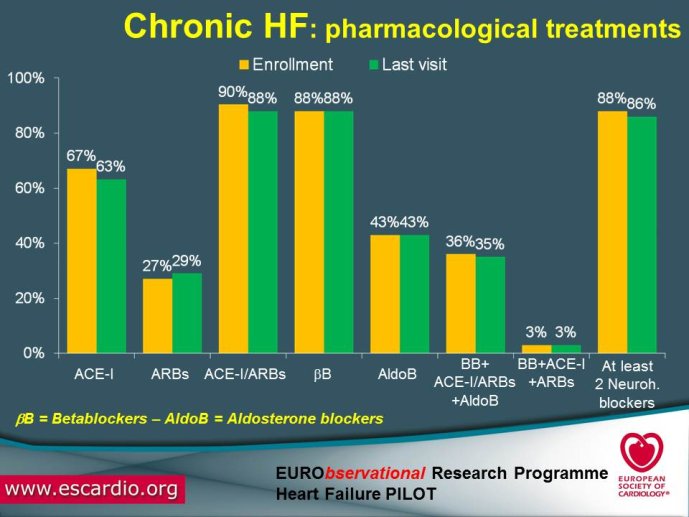
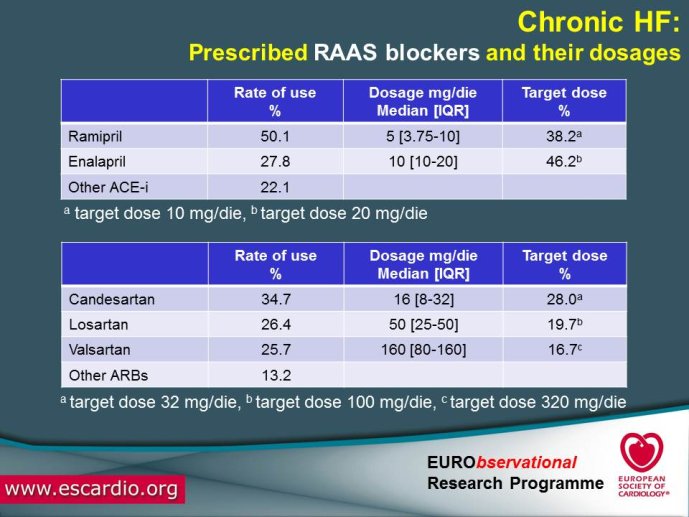
Chronic Heart Failure (HF) is associated with a high burden of mortality and morbidity, reduced quality of life, and increasing healthcare costs in both US and Europe. Evidence-based medicine represents the most effective means of ensuring that patients receive high-quality care and appropriate pharmacological/non-pharmacological management. With the increased prevalence of chronic HF, there is a concomitant increase in the number of related hospitalizations and, as chronic HF progresses, the risk of acute exacerbation increases. Acute HF is a complex, heterogeneous, clinical syndrome characterized by a rapid onset of signs and symptoms secondary to abnormal cardiac function, and it is often life threatening, requiring urgent therapy. In the United States, a primary diagnosis of acute HF accounts for more than one million hospitalizations each year, with similar numbers suggested for Europe. Despite significant advances in diagnosis and therapy obtained over the past 20 years, patients with HF, specifically those with acute HF, continue to have a poor long-term prognosis. Clinical destabilizations leading to hospitalization are associated with hemodynamic and neuro?hormonal alterations, which can contribute to progressive ventricular dysfunction and dilation, mitral regurgitation, increased wall stress, and progressive myocyte loss as a result of apoptosis and necrosis. Registries and surveys have been conducted in patients with either chronic HF or acute HF but a description of the whole clinical history of patients with HF, including the acute episodes and the consequent changes in clinical conditions and in the management strategies are not available. A registry able to capture all the relevant clinical information of patients with HF, including their acute episodes of decompensation, will enable us to improve our knowledge on epidemiology and outcomes of real world patients with this clinical condition. Further, specific questions of high clinical relevance could be answered using the information collected in the Registry. The ESC-HF Long-term Registry is a prospective,
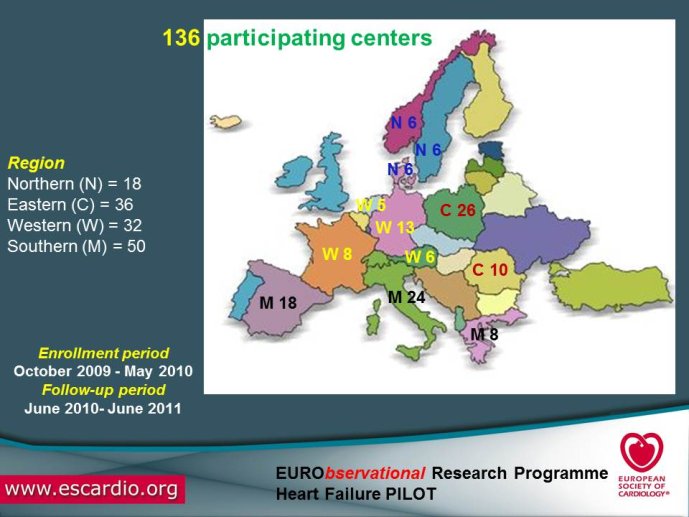
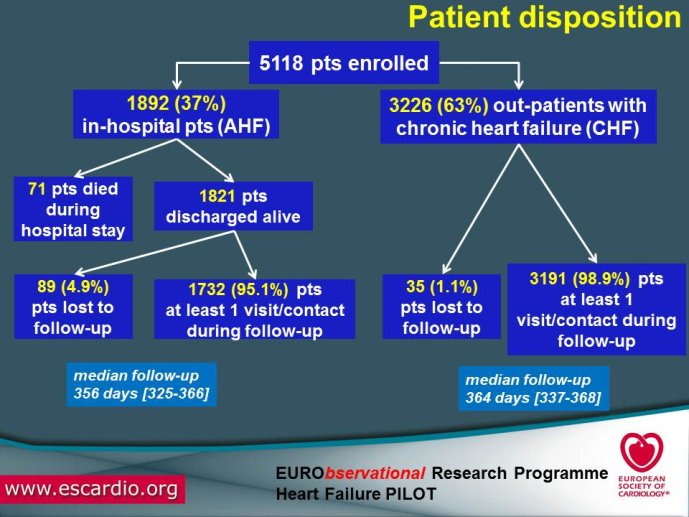
multicentre, observational study of patients presenting to Cardiology Centres in European countries. Site selection in each participating country will target a sample of hospitals of different levels of complexity from which patients will be recruited, focusing on capturing a broad spectrum of cardiology and HF specialty units regularly following outpatients with HF and admitting patients with acute, preexisting or new onset HF in order to build-up a network of centres representative of European reality. Up to the end of October 2012, more than 12,500 patients have been enrolled by 27 ESC countries.
L’auteur n’a pas transmis de conflit d’intérêt concernant les données diffusées dans cette vidéo ou publiées dans la référence citée.
9th Global Cardiovascular Clinical Trialists Forum • Paris 2012
Multidisciplinary expert workshop : achievements challenges and barriers to implantation of the ESC 2012 chronic heart failure guidelines.
Chairpersons: Alain COHEN-SOLAL, Paris, FRA - Adrian VOORS, Groningen, NED
Background: The ESC-HFA chronic and acute heart failure guidelines have recently been published. However, the challenge for guidelines does not cease with a consensus document. Practical implementation is the critical step in establishing higher standards of care for individual patients. Improved guideline uptake is not only an index of better standards but a validation of the process of guideline production.
Improving consensus between guidelines is also important, differences in recommendations may act as a barrier to guideline. The NICE CHF guidance was updated in 2010, and it is not likely to be revised in short term.
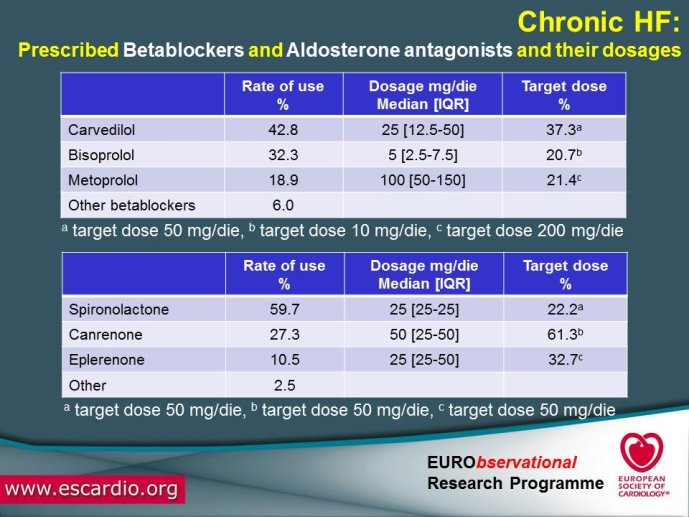
Practice differs from the guideline recommendations. Registries suggest differences in guideline interpretation and treatment/management of CHF between different stakeholders. Similarities and differences exist between GPs and hospital physicians’ approaches to management of CHF. One important issue that is not covered by the current guidelines is the class effect issue. Canadian and Australian CHF guideline and 2010 NICE guideline name eplerenone as preferred drug in heart failure, ESC mentions only mineralocorticoid receptor antagonists (MRAs.) as a class.
How to interpret compound vs. class effects while following guideline recommendations is an important issue.
Cost-effectiveness is a key not only to the content of guidelines but also in the assessment of implementation. Limits on healthcare resources mandate that resource-allocation decisions be guided by considerations of cost in relation to expected benefits. In cost-effectiveness analysis, the ratio of net healthcare costs to net health benefits provides an index by which priorities may be set.
Aims: This multidisciplinary consensus workshop aims at discussing CHF guideline implementation issues and the consequences on defining the place of MRA/eplerenone in management of CHF.
Réalisation, production : Canal U/3S et CERIMES
Keyword : Cardiovascular Clinical Trialists, Paris, 2012, Cardiovascular prevention, ESC-HFA, eplerenone
Dans la même collection
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
DELIARGYRIS Efthymios
MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Industry perspectiv…
WOEHRLE Holger
MODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
PRASAD Krishna
MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : Different doses, differe…
VERHEUGT Freek
MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Non randomized and/…
POCOCK Stuart J.
MODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
ZANNAD Faiez
MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : How to secure the optima…
GIBSON Michael
MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : Industry viewpoint (Joer…
KOECK Jean-Louis
MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 2 : Well Established Methods…
KOENIG Wolfgang
MODIGLIANI Workshop 2 - Friday November 30, 2012 : ATHEROSCLEROSIS IMAGING IN CLINICAL TRIALS Facilitating the discovery of effective therapies Chairpersons: Jagat NARULA, New York, USA - Ahmed
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Options of and alte…
ABRAHAM William T.
MODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : How to secure the optima…
GELLER Nancy L.
MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Debate Session 5 : Ultrafiltration fo…
ROSSI Gian Paolo
MODIGLIANI Debate Session 5 - Saturday December 1st, 2012 NOVEL DIURETIC STRATEGIES IN HEART FAILURE Chairpersons: Keld KJELDSEN, Copenhagen, DEN - Gian Paolo ROSSI, Padua, ITA Webcast: Patrick