Notice
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 2 : Well Established Methods for Imaging Approaches, IVUS and IMT (Wolfgang KOENIG)
- document 1 document 2 document 3
- niveau 1 niveau 2 niveau 3
Descriptif
MODIGLIANI Workshop 2 - Friday November 30, 2012 :
ATHEROSCLEROSIS IMAGING IN CLINICAL TRIALS
Facilitating the discovery of effective therapies
Chairpersons: Jagat NARULA, New York, USA - Ahmed TAWAKOL, Boston, USA
Webcast: Bart STAELS, Lille, FRA
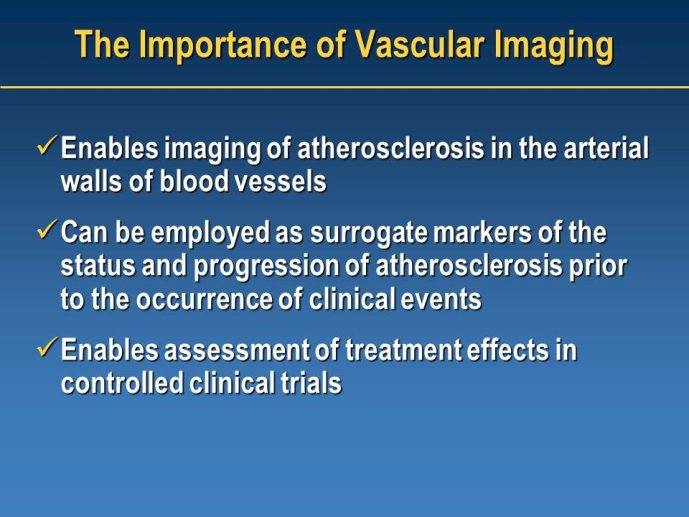
Phase III clinical endpoint trials evaluating treatments for atherosclerosis typically require very large sample sizes, cost hundreds of millions of dollars and historically have had very low success rates. As a result, few new therapies that attenuate the progression of atherosclerosis have been identified in over 30 years (since the discovery of statins).
Nearly a decade ago, in recognition of the low success of Phase III trials, regulatory agencies called for the adoption of new biomarkers or surrogate endpoints to enhance the rate of clinical development. To that end, several cardiovascular imaging technologies have gone through evolutionary cycles of validation over the past decade and several have demonstrated promise as clinical tools and as clinical trial biomarkers.
With the rapid development and implementation of these imaging approaches, it is important to delineate the opportunities and limitations associated with these tools. In particular, it is essential to identify imaging biomarkers that might accurately predict eventual clinical success based on the observed changes in the atherosclerotic imaging measurements. With such tools as gatekeepers, only those treatments with proven efficacy during Phase II trials would be promoted to Phase III
with the expectation of high likelihood of success in the clinical endpoint trials. By enhancing the success rate of Phase III clinical trials, use of these imaging tools have the potential to accelerate the discovery of treatments for atherosclerosis.
Session program:
Overview: Why are imaging endpoints needed in CV clinical trials
Speaker: Jagat NARULA, New York, USA
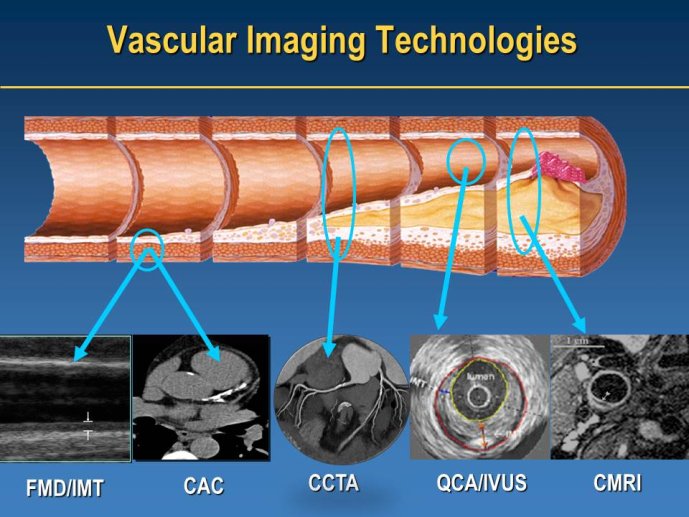
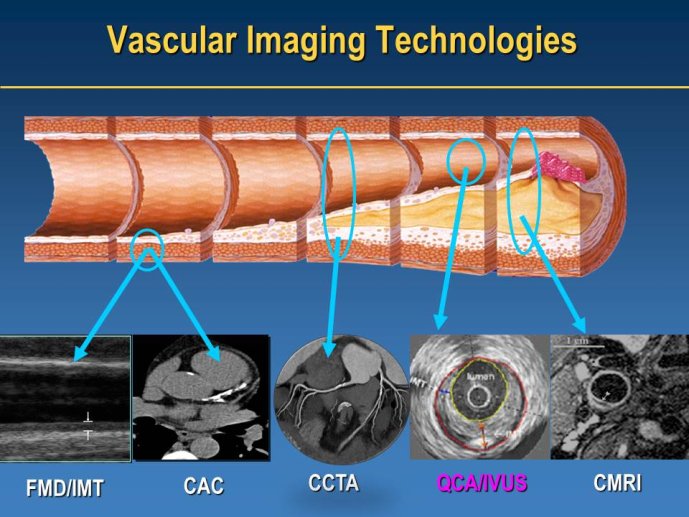
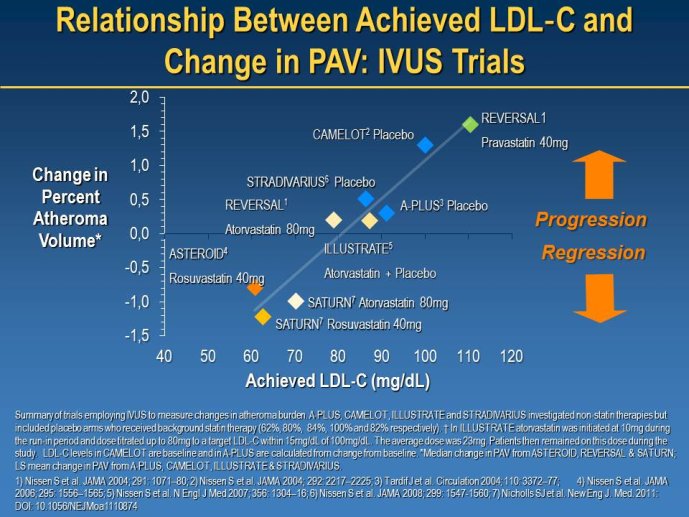
Well Established Methods for Imaging Approaches: IVUS and IMT
Speaker: Jean-Claude TARDIF, Montréal, CAN
Discussant: Wolfgang KOENIG, Ulm, GER
Coronary CTA in clinical trials
Speaker: Udo HOFFMANN, Boston, USA
MRI imaging in clinical trials
Speaker: Zahi FAYAD, New York, USA
Discussant: Robin CHOUDHURY, Oxford, GBR
PET-CT imaging in clinical trials
Speaker: Ahmed TAWAKOL, Boston, USA
Discussant: James RUDD, Cambridge, GBR
Intervention / Responsable scientifique
Thème
Documentation
Liens
Dans la même collection
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
DeliargyrisEfthymiosMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Industry perspectiv…
WoehrleHolgerMODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
PrasadKrishnaMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : Different doses, differe…
VerheugtFreekMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Non randomized and/…
PocockStuart J.MODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
ZannadFaiezMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : How to secure the optima…
GibsonMichaelMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : Industry viewpoint (Joer…
KoeckJean-LouisMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Options of and alte…
AbrahamWilliam T.MODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : How to secure the optima…
GellerNancy L.MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Debate Session 5 : Insights from DOSE…
MascetteAliceMODIGLIANI Debate Session 5 - Saturday December 1st, 2012 NOVEL DIURETIC STRATEGIES IN HEART FAILURE Chairpersons: Keld KJELDSEN, Copenhagen, DEN - Gian Paolo ROSSI, Padua, ITA Webcast: Patrick
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Debate Session 5 : The Vaptans story …
AbrahamWilliam T.MODIGLIANI Debate Session 5 - Saturday December 1st, 2012 NOVEL DIURETIC STRATEGIES IN HEART FAILURE Chairpersons: Keld KJELDSEN, Copenhagen, DEN - Gian Paolo ROSSI, Padua, ITA Webcast: Patrick
Avec les mêmes intervenants et intervenantes
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 5 : Identifying new targets:…
KoenigWolfgangWill new compounds be able to reduce the residual risk in high risk patients when treatment targets based on new ESC guidelines have been achieved (e.g. LDL-C lesser than 70mg/dl)?
-
Cardiovascular Clinical Trialists (CVCT) Forum - Paris 2012 : Target populations: How do we risk-st…
KoenigWolfgangTitle : Cardiovascular Clinical Trialists (CVCT) Forum - Paris 2012 : Target populations: How do we risk-stratify? Are additional biomarkers helpful? Speaker: Wolfgang KOENIG, Ulm, GER Discussant:
Sur le même thème
-
La naissance de la médecine scientifique (par Pierre Corvol)
CorvolPierreMontenotJeanLa naissance de la médecine scientifique Dans La Maison Nuncingen (1837), Balzac met en scène une conversation entre quatre journalistes échauffés par un bon repas. L’un des commensaux, Émile
-
Prévention de l'accident vasculaire cérébral
Chaque année, en France, près de 125 000 cas d'AVC -- Accident Vasculaire Cérébral -- sont recensés. Avec les récidives, ce chiffre augmente de 25 % L' AVC représente la 1ère cause de handicap et
-
Du coté de chez...Michel Haïssaguerre
GloinecYvesPreNadègeInspiré du questionnaire de Proust, cet entretien plus intimiste engage une réflexion sur Michel Haïssaguerre en tant qu’homme et non plus en tant que cardiologue. Ce face-à-face permet de
-
Michel Haïssaguerre, entre rythmes et musicalité
GloinecYvesPreNadègeA l’occasion de la création de l’Institut Hospitalo-Universitaire LIRYC (Institut de Rythmologie et de Modélisation Cardiaque) dont il est à l’origine, le 2e volet de notre série Trip TIC
-
Cardio-vasculaire
GayBernardGossePhilippeDouardHervéSassoustGérardJournées Bordeaux Segalen 2013 - Formation Médicale Continue des Médecins Généralistes - Session Cardio-vasculaire
-
Échographie de contraste
LafitteStéphaneCottarre-LafitteMarianneRéantPatriciaRoudautRaymondCe cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,
-
Infarctus du myocarde
LafitteStéphaneCottarre-LafitteMarianneRéantPatriciaRoudautRaymondCe cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,
-
Prothèses valvulaires
LafitteStéphaneCottarre-LafitteMarianneRéantPatriciaRoudautRaymondCe cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,
-
Néoformations cardiaques
LafitteStéphaneCottarre-LafitteMarianneRéantPatriciaRoudautRaymondCe cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,
-
Complications de l'infarctus du myocarde
LafitteStéphaneCottarre-LafitteMarianneRéantPatriciaRoudautRaymondCe cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,
-
Les endocardites
LafitteStéphaneCottarre-LafitteMarianneRéantPatriciaRoudautRaymondCe cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,
-
Myocardiopathies dilatées
LafitteStéphaneCottarre-LafitteMarianneRéantPatriciaRoudautRaymondCe cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,