Notice
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is heart failure a viable new potential indication for anti-thrombosis therapy (Faiez ZANNAD)
- document 1 document 2 document 3
- niveau 1 niveau 2 niveau 3
Descriptif
MODIGLIANI Workshop 1 - Friday November 30, 2012 :
THE THROMBOSIS TRIALISTS WORKSHOP
DOSE AND TARGET PATIENT POPULATIONS ISSUES
Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN, Bucharest, ROM
Webcast: Johanne SILVAIN, Paris, FRA
The development of new antithrombotic agents is a challenging area of cardiovascular medicine.
These agents generally have a narrow therapeutic margin, and it is often difficult to find the optimal balance between efficacy (reduction of ischemic events) and safety (no excess of bleeding events).
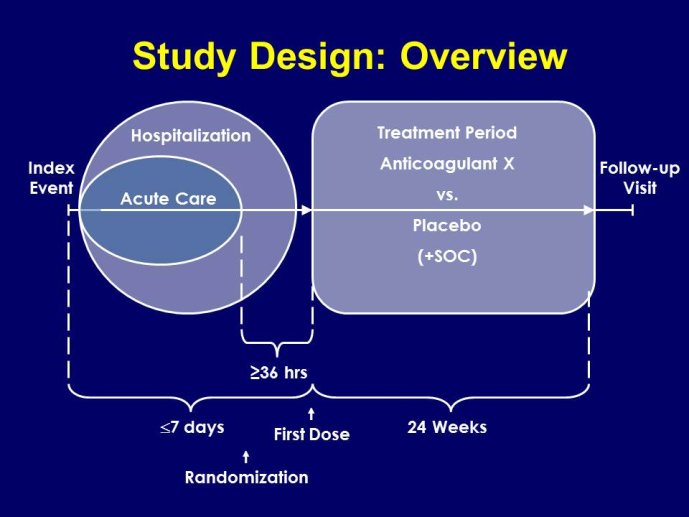
➢ Phase II trials are designed to generate signals of safety and efficacy and to select the dose or doses for phase III pivotal trials. However, phase II data are limited by small numbers of events and short follow-up. Some recent phase III trials have produced results that were unanticipated from the phase II findings.
➢ Adaptive designs to explore safety (bleeding) risks have been proposed to overcome the current limitations of dose selection for phase III. Several concerns with these designs remain unresolved, such as appropriate methods to control Type I error for both safety and efficacy. Also, adaptive designs will only be useful if they are accepted by the regulatory agencies. The agencies have not announced a final position yet. Implementation of adaptive designs requires early and in-depth interaction between the agencies and study sponsors.
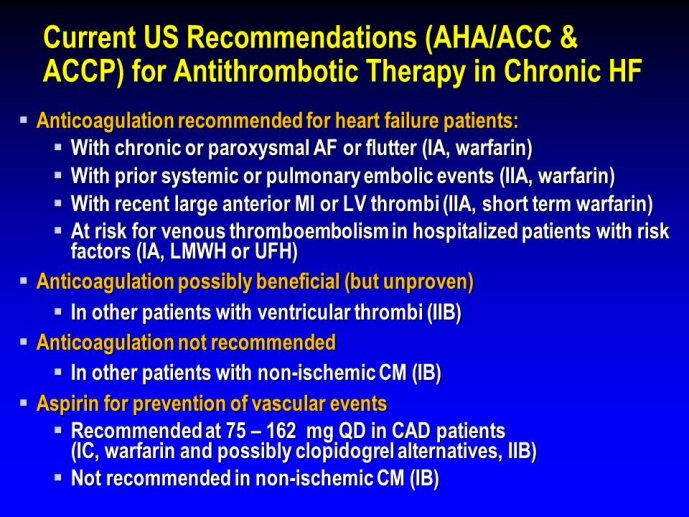
➢ With the newer anti-thrombotic agents progressively potentially replacing warfarin, we are moving from a target INR guided dosing to an indication/risk guided dosing. Different doses are being developed for different indications. There is an intense discussion on whether doses of anticoagulants should be similar or different for preventing DVT after orthopedic surgery, for treating DVT and pulmonary embolism, for AFib, for prevention after ACS and for prevention in artificial valves. Dose - effect relationships are difficult to establish. A balance should be found between the dose relationship with clinical benefit and the dose relationship with the risk of bleeding. An intense discussion is also ongoing on whether a range of doses rather than one single dose should be made available for the same disease.
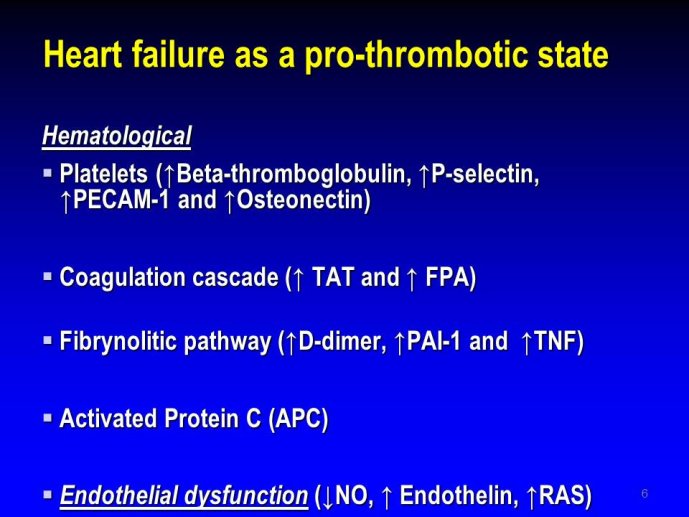
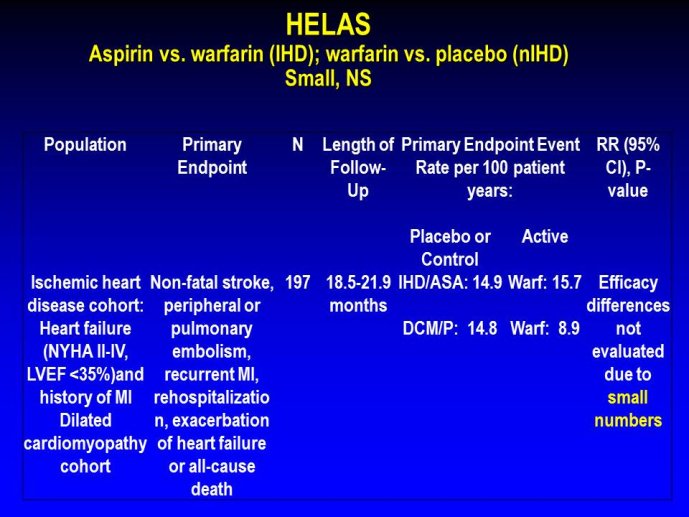
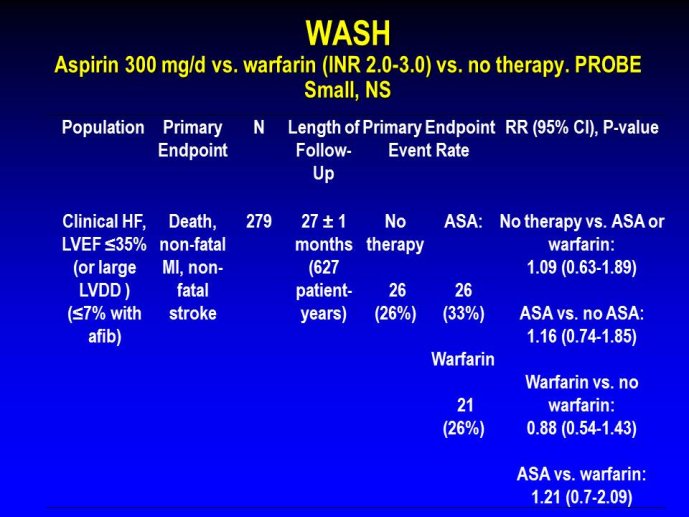
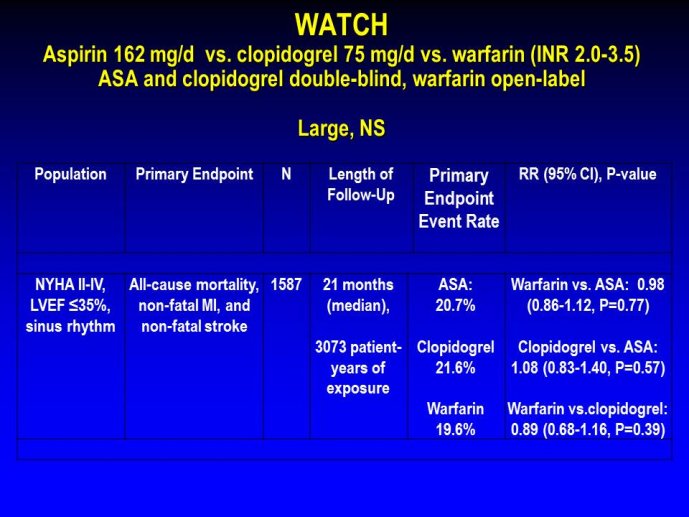
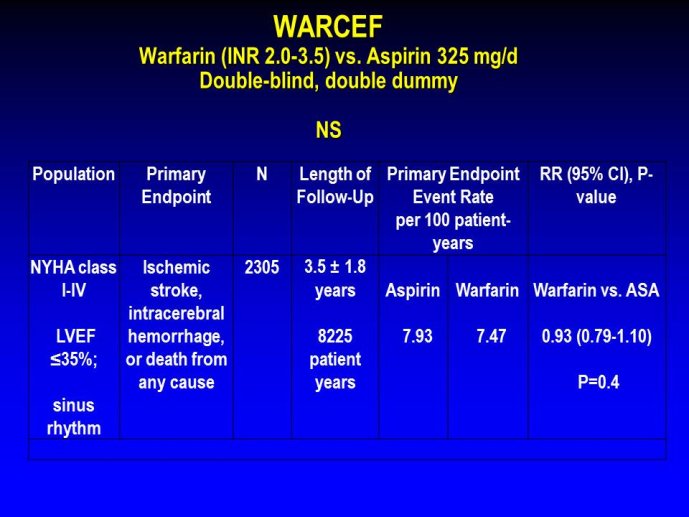
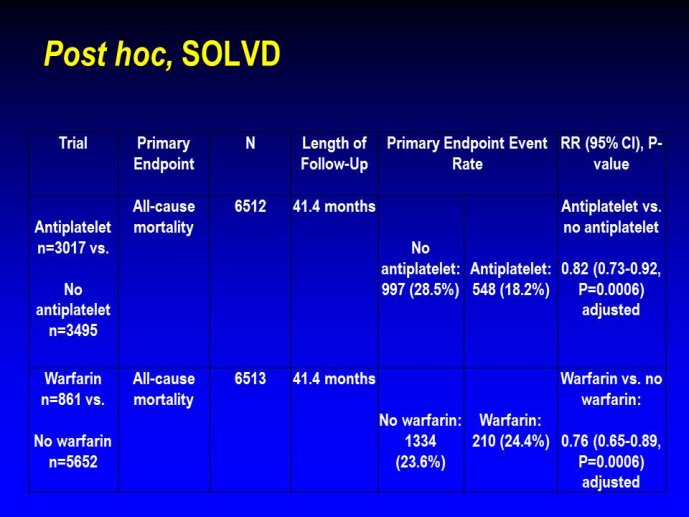
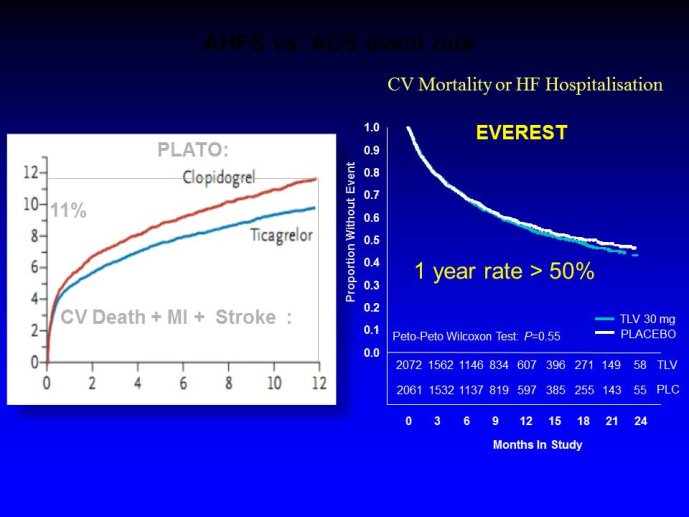
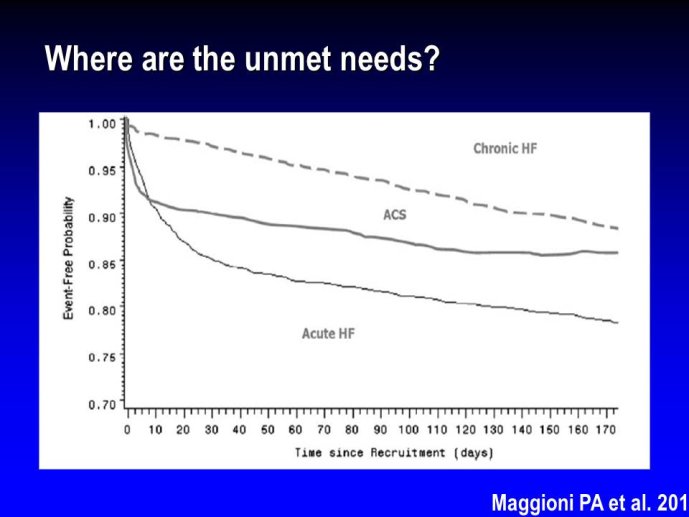
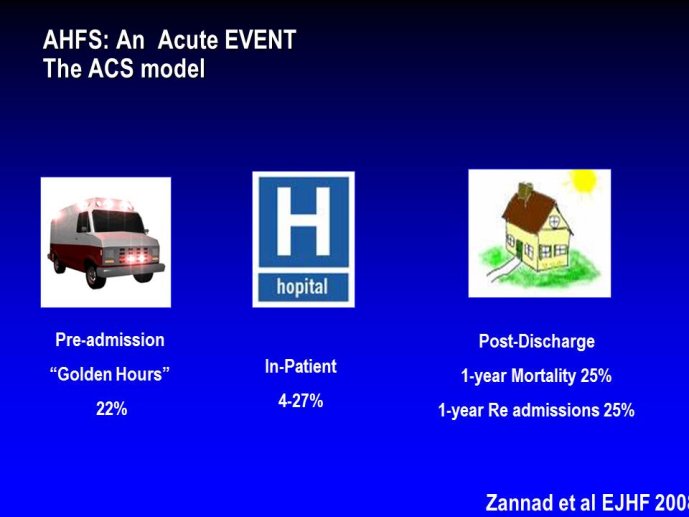
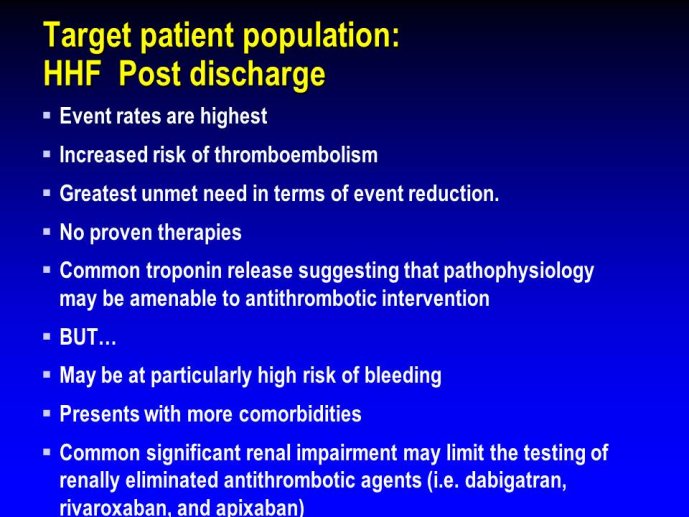
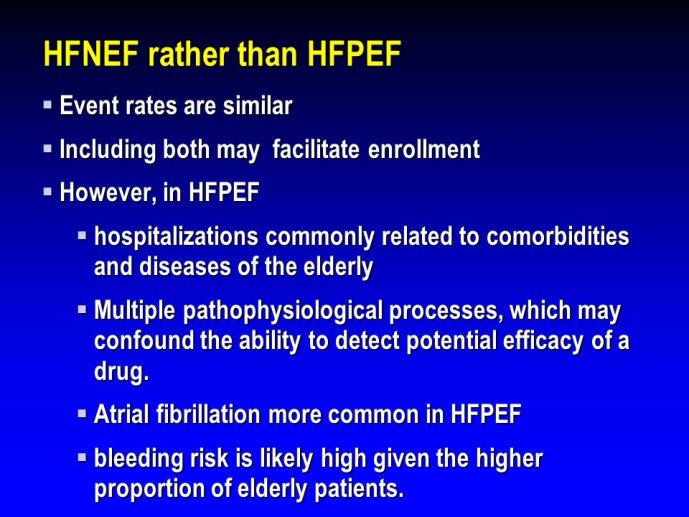
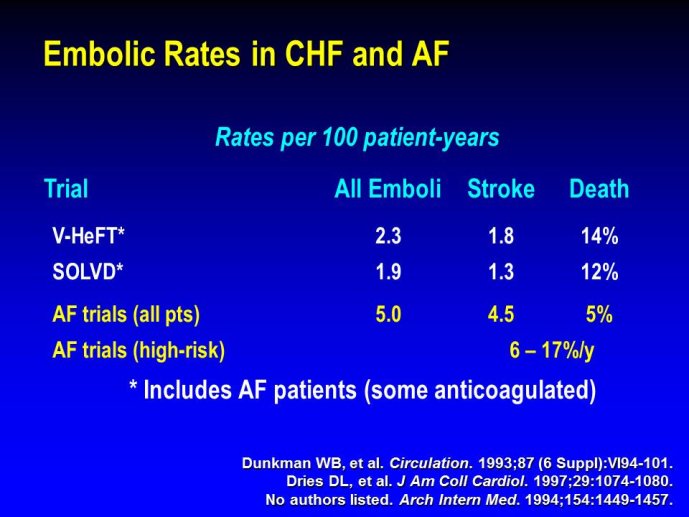
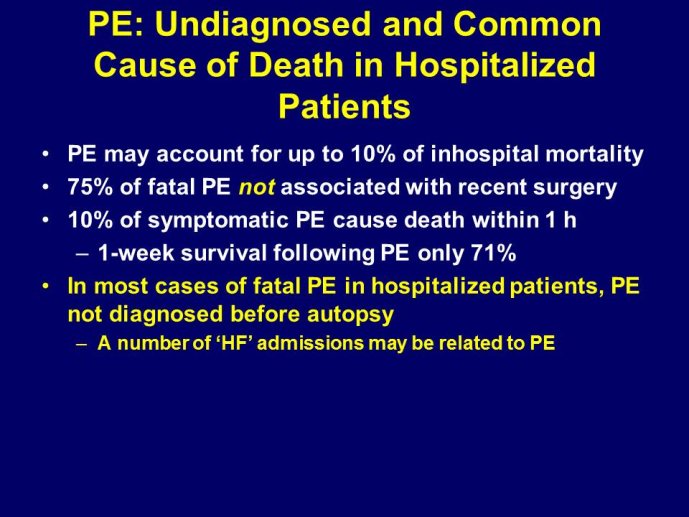
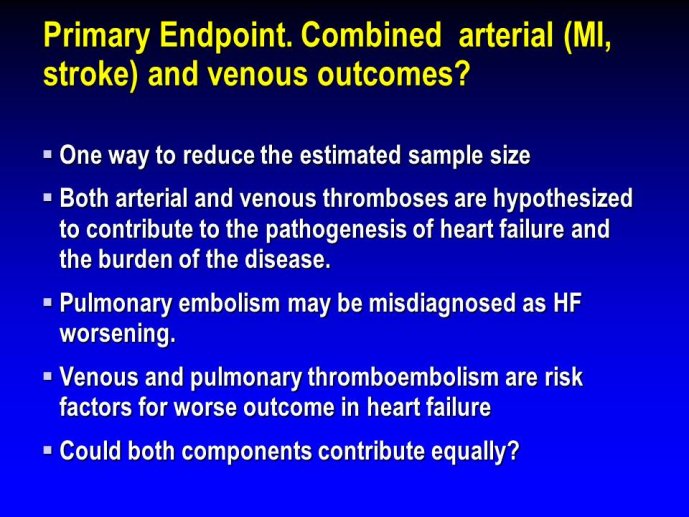
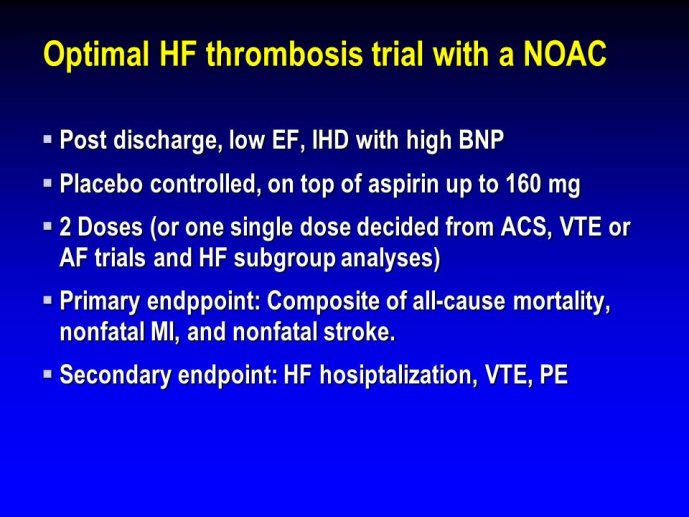
➢ Exploring new indications for the newer anti-thrombotic agents might be one target of interest in patients with heart failure. In patients with coronary artery disease and HF, a majority of deaths (including sudden death) appear to be related to ischemia and worsening of heart failure. The presence of pulmonary embolism as a cause of worsening HF is underestimated and certain underlying pathophysiological mechanisms are common to both arterial and venous thrombi. It has been recognized since long that HF is associated with hypercoagulable state, and, at the cellular level, it has been reported that thrombin exerts multiple actions on cardiomyocytes which can favor the genesis of arrhythmias and myocyte injury. Antithrombotic strategies to reduce the risk of death, myocardial infarction (MI), or stroke have been tested in several randomized trials. These studies were underpowered and the recently published WARCEF trial is inconclusive. With the newest
ant-thrombotic agents being probably safer, heart failure might be one of the next diseases where to expand the indications of new antithrombotics. Designing an anti-thrombotic trial in HF might also yield meaningful data regarding the potential role of thrombosis in patients with heart failure.
The aim of this session is to explore novel, scientifically rigorous methodologies through an open, cooperative dialogue among investigators, regulators, and sponsors.
Session program:
Dosing issues
• How to secure the optimal dose(s) for phase III? Can adaptive design help?
Speaker: Nancy GELLER, NHLBI, USA
Discussant: Michael GIBSON, Boston, USA
• Different doses, different indications? DVT and pulmonary embolism, Atrial fibrillation, ACS, artificial valves
Speaker: Freek VERHEUGT, Amsterdam, NED
Discussant ACS: Maarten SIMOONS, Rotterdam, NED
Industry viewpoint: Scott BERKOWITZ, Bayer, USA - Christophe GAUDIN, Sanofi, FRA - Lloyd HASKEL, J&J, USA - Yasser KHDER, Boehringer Ingelheim, FRA - Joerg KOGLIN, Merck, USA - John LAWRENCE, BMS, USA
Regulatory viewpoint: Angeles ALONSO, EMEA, ESP - Pieter DE GRAEF, EMEA, NED - Kaori SHINAGAWA, PMDA, JAP
New indications: Is heart failure a viable new potential indication for anti-thrombosis therapy
Speaker: Faiez ZANNAD, Nancy, FRA
Discussants: Efthymios DELIARGYRIS, MedCo, USA - Lloyd HASKEL, J&J, USA
Regulatory viewpoint: Krishna PRASAD, MHRA, GBR
Thème
Documentation
Liens
Dans la même collection
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : Industry viewpoint (Joer…
KoeckJean-LouisMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : Different doses, differe…
VerheugtFreekMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Industry perspectiv…
WoehrleHolgerMODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
PrasadKrishnaMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : How to secure the optima…
GibsonMichaelMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Non randomized and/…
PocockStuart J.MODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
DeliargyrisEfthymiosMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Options of and alte…
AbrahamWilliam T.MODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : How to secure the optima…
GellerNancy L.MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 2 : Well Established Methods…
KoenigWolfgangMODIGLIANI Workshop 2 - Friday November 30, 2012 : ATHEROSCLEROSIS IMAGING IN CLINICAL TRIALS Facilitating the discovery of effective therapies Chairpersons: Jagat NARULA, New York, USA - Ahmed
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Debate Session 5 : The Vaptans story …
FelkerG. MichaelMODIGLIANI Debate Session 5 - Saturday December 1st, 2012 NOVEL DIURETIC STRATEGIES IN HEART FAILURE Chairpersons: Keld KJELDSEN, Copenhagen, DEN - Gian Paolo ROSSI, Padua, ITA Webcast: Patrick
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Debate Session 5 : Insights from DOSE…
MascetteAliceMODIGLIANI Debate Session 5 - Saturday December 1st, 2012 NOVEL DIURETIC STRATEGIES IN HEART FAILURE Chairpersons: Keld KJELDSEN, Copenhagen, DEN - Gian Paolo ROSSI, Padua, ITA Webcast: Patrick
Avec les mêmes intervenants et intervenantes
-
Semaine Médicale de Lorraine Nancy 2012 – Hypertension artérielle. Quelle prise en charge ?
ZannadFaiezT0403-06 Titre : Semaine Médicale de Lorraine Nancy 2012 – Hypertension artérielle. Quelle prise en charge ? Intervenant (e)(s) : Faiez ZANNAD (service de Cardiologie – CHU Nancy Brabois) Résumé :
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 : Omics research and system biology. Ke…
ZannadFaiezHow future trials may help optimizing benefit-to-risk ratio. The role of specialist scientific organizations. ISCP: Speaker: Felipe MARTINEZ, Cordoba, ARG CVCT: Speaker: Faiez ZANNAD, Nancy, FRA ESC
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 : Autonomic modulation therapy for hear…
ZannadFaiezTitle : Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 : Autonomic modulation therapy for heart failure: Preclinical data and ongoing trials Speaker: Faiez ZANNAD, Nancy, FRA Discussant:
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 : Impact of major clinical trials on ES…
ZannadFaiezTitle : Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 : Impact of major clinical trials on ESC Chronic Heart Failure 2012 guidelines. Game changer trials: EMPHASIS-HF, SHIFT, Devices…
-
Forum Sante : Vers un coeur immortel
KhalifeKhaliféZannadFaiezForum Santé : Vers un coeur immortel ?
-
Semaine de la Recherche Thérapeutique 26-03-09 Faïez ZANNAD
ZannadFaiezSemaine de la Recherche Thérapeutique, Faëz ZANNAD, Diabète, Cholestérol, Obésité
-
Semaine de la recherche thérapeutique - Faïez ZANNAD
ZannadFaiezConférence de Faïez ZANNAD pour le Débat Décideur
-
10ème colloque de biologie prospective-Epaisseur Intima-Média
ZannadFaiezLe complexe entre l'intima et la média est l'endroit ou se développe l'artéro-sclérose . L'épaisseur intima-media se produit plus tardivement chez les femmes que chez les hommes . Les facteurs de
Sur le même thème
-
La naissance de la médecine scientifique (par Pierre Corvol)
CorvolPierreMontenotJeanLa naissance de la médecine scientifique Dans La Maison Nuncingen (1837), Balzac met en scène une conversation entre quatre journalistes échauffés par un bon repas. L’un des commensaux, Émile
-
Prévention de l'accident vasculaire cérébral
Chaque année, en France, près de 125 000 cas d'AVC -- Accident Vasculaire Cérébral -- sont recensés. Avec les récidives, ce chiffre augmente de 25 % L' AVC représente la 1ère cause de handicap et
-
Michel Haïssaguerre, entre rythmes et musicalité
GloinecYvesPreNadègeA l’occasion de la création de l’Institut Hospitalo-Universitaire LIRYC (Institut de Rythmologie et de Modélisation Cardiaque) dont il est à l’origine, le 2e volet de notre série Trip TIC
-
Du coté de chez...Michel Haïssaguerre
GloinecYvesPreNadègeInspiré du questionnaire de Proust, cet entretien plus intimiste engage une réflexion sur Michel Haïssaguerre en tant qu’homme et non plus en tant que cardiologue. Ce face-à-face permet de
-
Cardio-vasculaire
GayBernardGossePhilippeDouardHervéSassoustGérardJournées Bordeaux Segalen 2013 - Formation Médicale Continue des Médecins Généralistes - Session Cardio-vasculaire
-
Échocardiographie trans-œsophagienne
LafitteStéphaneCottarre-LafitteMarianneRéantPatriciaRoudautRaymondCe cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,
-
Échocardiographie-Doppler normale
LafitteStéphaneCottarre-LafitteMarianneRéantPatriciaRoudautRaymondCe cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,
-
Indications de l'ETO
LafitteStéphaneCottarre-LafitteMarianneRéantPatriciaRoudautRaymondCe cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,
-
Rétrécissement mitral
LafitteStéphaneCottarre-LafitteMarianneRéantPatriciaRoudautRaymondCe cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,
-
Dissection aortique
LafitteStéphaneCottarre-LafitteMarianneRéantPatriciaRoudautRaymondCe cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,
-
Syndrome d'aorte douloureuse
LafitteStéphaneCottarre-LafitteMarianneRéantPatriciaRoudautRaymondCe cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,
-
Hypertension artérielle
LafitteStéphaneCottarre-LafitteMarianneRéantPatriciaRoudautRaymondCe cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,