Notice
Cardiovascular Clinical Trialists (CVCT) Forum - Paris 2012 : Non-inferiority, superiority, or both? Operationalizing the FDA guidance
- document 1 document 2 document 3
- niveau 1 niveau 2 niveau 3
Descriptif
Title :Cardiovascular Clinical Trialists (CVCT) Forum - Paris 2012 :
Non-inferiority, superiority, or both? Operationalizing the FDA guidance
Speaker: Marc PFEFFER, Boston, USA
Discussant: Stuart POCOCK, London, GBR
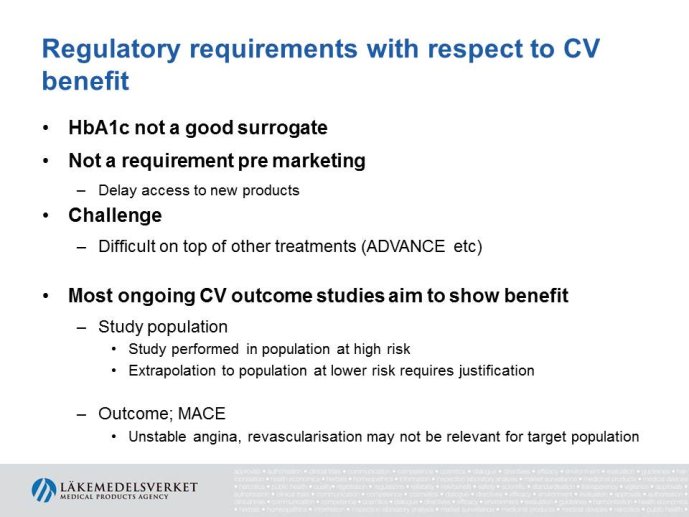
Regulatory viewpoint: Kristina DUNDER, EMEA, SWE
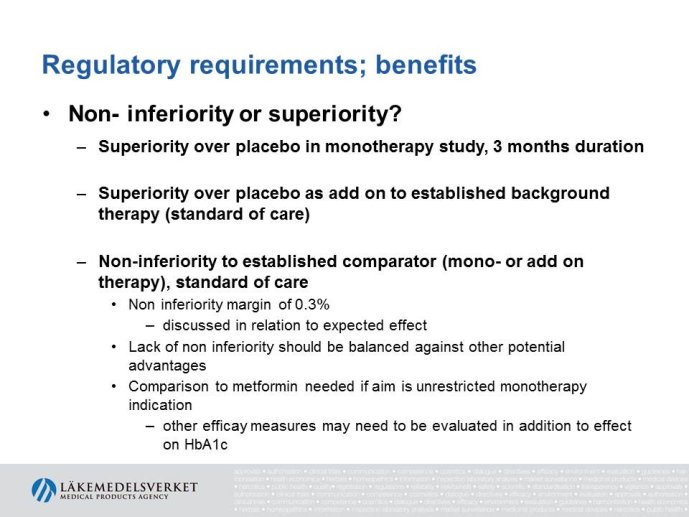
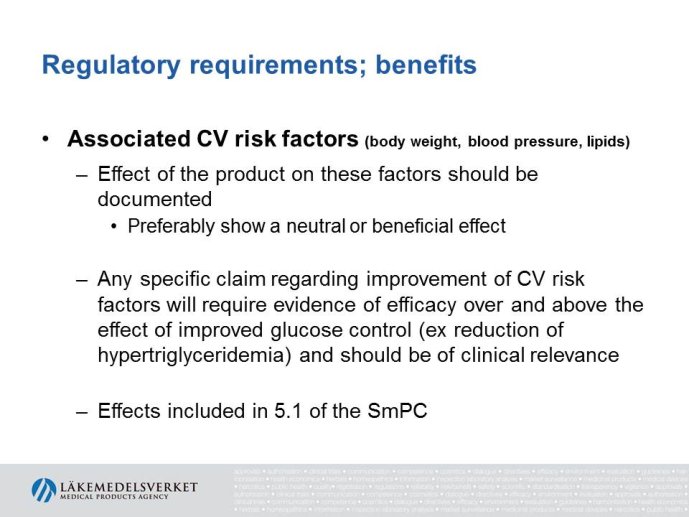
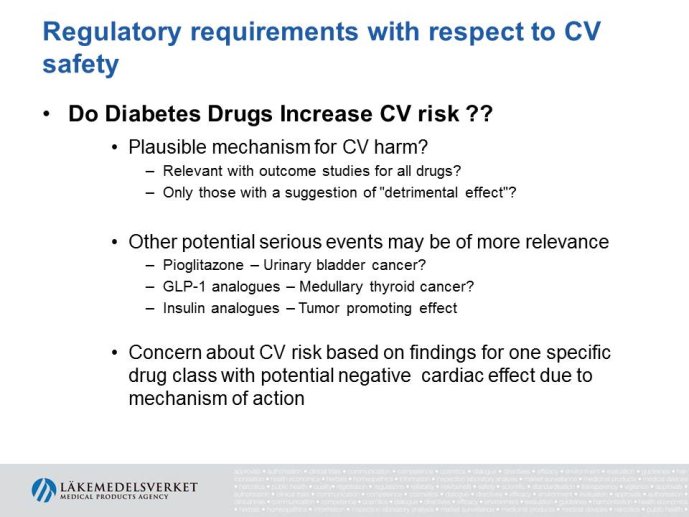
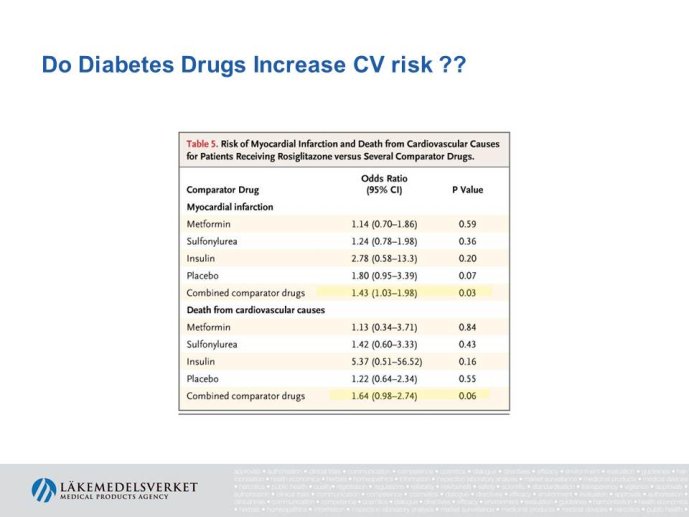
Abstract : The evaluation of cardiovascular safety of rosiglitazone (2006-2010) had implications on regulatory guidance for glucose lowering drugs both in the EU and in the US and both the FDA and the CHMP guidelines for development of glucose lowering drugs have been updated including new requirements for assessment of cardiovascular safety. While the FDA Guidance is very precise specifying that the upper bound of the 95% confidence interval for the estimated risk should be below 1.8 at the time of marketing application, the CHMP guidance focuses more on the
totality of data as a base for assessing the cardiovascular risk.
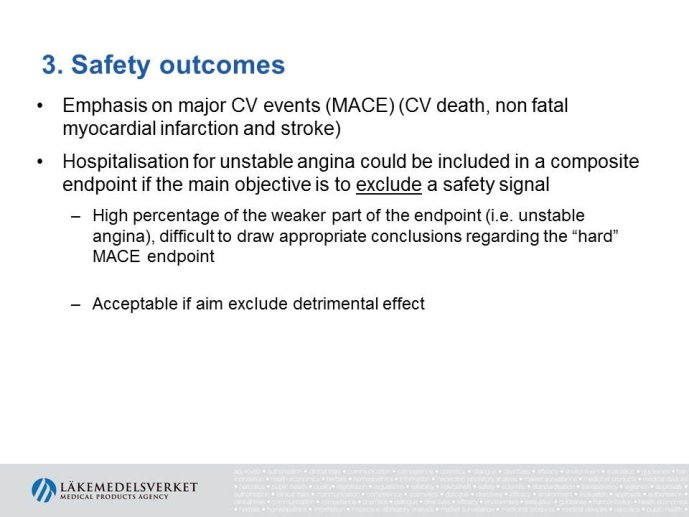
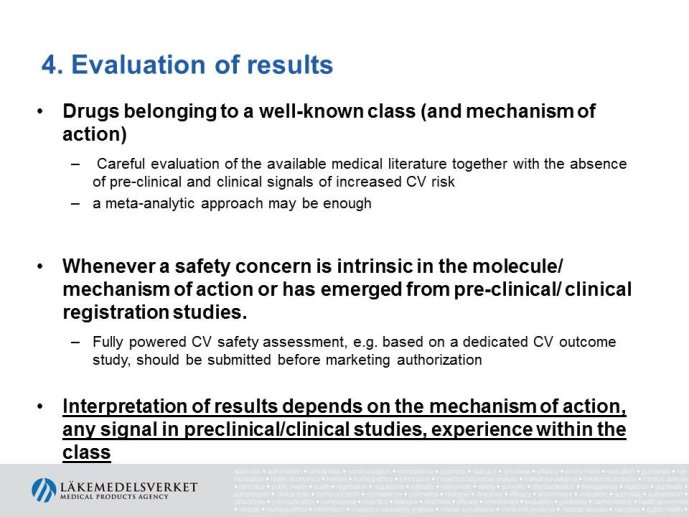
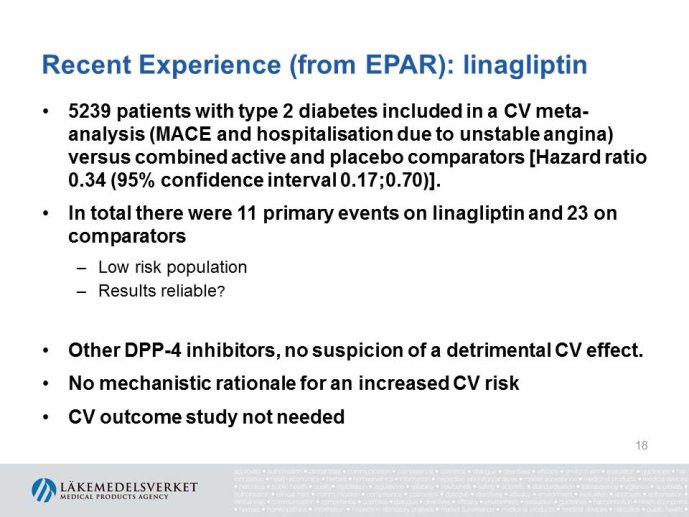
The presentation will focus on the interpretation of the requirements in the CHMP guideline (type of studies, study population, outcome measures, evaluation of the results) as well as experience from recent applications for marketing authorization for glucose lowering drugs, e.g. the usefulness of MACE meta analyses and the need for CV outcome studies for all new drugs.
L’auteur n’a pas transmis de conflit d’intérêt concernant les données diffusées dans cette vidéo ou publiées dans la référence citée.
Conférence enregistrée : 9th Global Cardiovascular Clinical Trialists Forum • Paris 2012
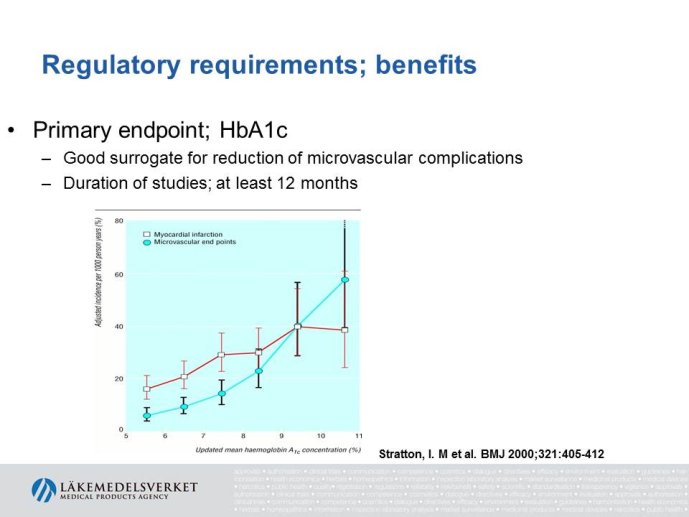
Diabetes clinical trials : helped or hindered by the current shift in regulatory requirements? Glycaemic control is an inadequate surrogate marker of cardiovascular event reduction in patients with type 2 diabetes. Clinical trials to date have been unsuccessful in identifying a therapeutic approach that addresses the underlying problem in diabetes (glycaemic control) and reduces cardiovascular risk. The potential for some agents to increase the risk of cardiovascular events has led to substantial changes in regulatory requirements for new anti-diabetic therapies. These requirements, while key to ensuring the cardiovascular safety of new agents, fail to emphasize the need to show clinical benefits, such as less visual impairment, less need for dialysis, or fewer cardiovascular events and deaths. Changes in test results such as glycaemic control, serum creatinine, micro-albuminuria, or retinopathy are inadequate surrogates. Regulators should consider the potential advantages of offering extended patent protection in order to encourage companies to conduct long-term trials in diabetes and many other chronic medical conditions. Cooperative efforts among physicians, clinical trialists, regulators, and sponsors are needed to address unresolved issues including re-defining therapeutic targets that are meaningful to patients with diabetes, determining the appropriate length of follow-up for future trials, and considering the ethical and operational challenges of non-inferiority designs.
Chairpersons: Marc PFEFFER, Boston, USA - Kausik RAY, London, GBR
Réalisation, production : Canal U/3S et CERIMES
Keyword : Cardiovascular Clinical Trialists, Paris, 2012, Cardiovascular prevention, Baro-stimulation, diabetes
Intervention / Responsable scientifique
Dans la même collection
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
ZannadFaiezMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : How to secure the optima…
GibsonMichaelMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : Industry viewpoint (Joer…
KoeckJean-LouisMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
DeliargyrisEfthymiosMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Industry perspectiv…
WoehrleHolgerMODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
PrasadKrishnaMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : Different doses, differe…
VerheugtFreekMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Non randomized and/…
PocockStuart J.MODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : How to secure the optima…
GellerNancy L.MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 2 : Well Established Methods…
KoenigWolfgangMODIGLIANI Workshop 2 - Friday November 30, 2012 : ATHEROSCLEROSIS IMAGING IN CLINICAL TRIALS Facilitating the discovery of effective therapies Chairpersons: Jagat NARULA, New York, USA - Ahmed
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Options of and alte…
AbrahamWilliam T.MODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Debate Session 5 : The Vaptans story …
AbrahamWilliam T.MODIGLIANI Debate Session 5 - Saturday December 1st, 2012 NOVEL DIURETIC STRATEGIES IN HEART FAILURE Chairpersons: Keld KJELDSEN, Copenhagen, DEN - Gian Paolo ROSSI, Padua, ITA Webcast: Patrick