Notice
Cardiovascular Clinical Trialists (CVCT) Forum - Paris 2012 : Target populations: How do we risk-stratify? Are additional biomarkers helpful?
- document 1 document 2 document 3
- niveau 1 niveau 2 niveau 3
Descriptif
Title : Cardiovascular Clinical Trialists (CVCT) Forum - Paris 2012 :
Target populations: How do we risk-stratify? Are additional biomarkers helpful?
Speaker: Wolfgang KOENIG, Ulm, GER
Discussant: Adrian HERNANDEZ, Durham, USA
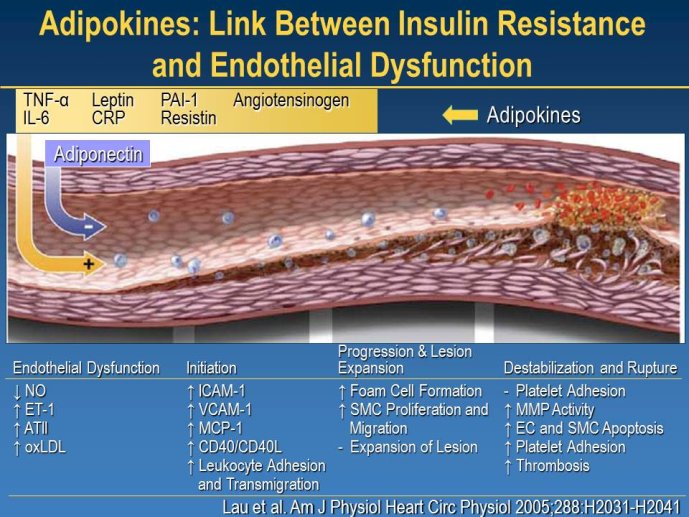
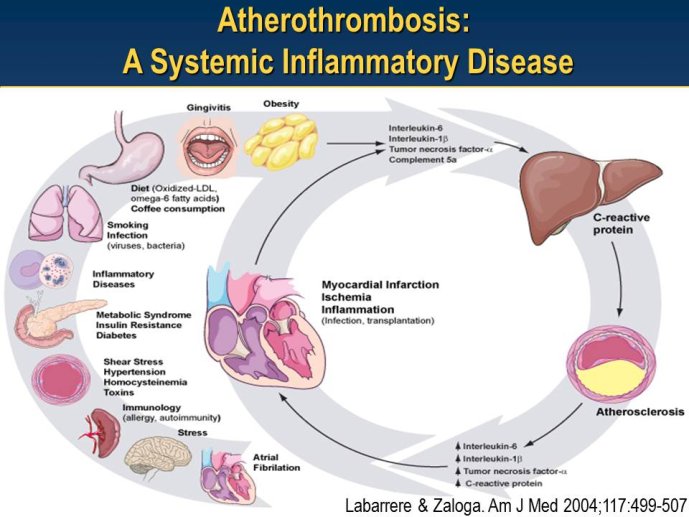
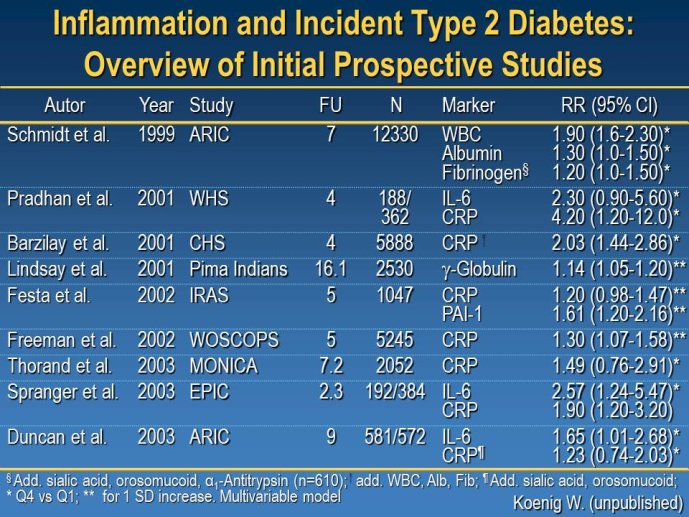
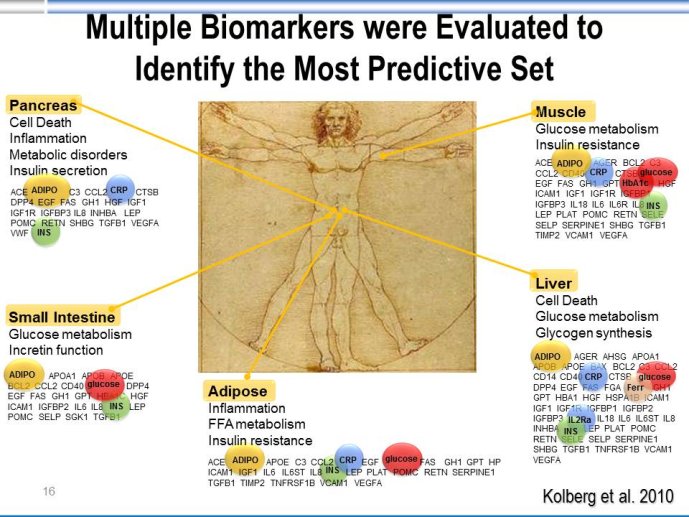
Abstract : LDL cholesterol (LDL-C) is causally involved in the atherosclerotic process and presents the major lipid variable for risk assessment, and statin therapy is guided by different levels of target LDL-C according to the absolute risk of the patient. Statins have revolutionized the treatment of hypercholesterolemia in patients without but also with manifest atherosclerosis and numerous trials since 4S have consistently documented a decrease in cardiovascular risk associated with decreases in LDL-C. Thus, based on meta-analyses a decrease of LDL-C by 40 mg/dl or approx. 1 mmol/L is associated with a 22% decrease in vascular endpoints. Overtime, lower targets for LDL-C have been defined based on the results of randomized clinical trials but also on evidence from IVUS studies. A most recent large IVUS study comparing the two most potent statins atorvastatin 40 mg and atorvastatin 80 mg showed regression of atherosclerosis in almost 2/3 of the subjects who had achieved LDL-C levels between 60 and 70 mg/ dl. A meta-analysis of all available IVUS trials has shown that regression of atherosclerosis seems feasible once the LDL-C target is below 80 mg/dl. However, even the most potent statins in the highest dose (atorvastatin 80 mg or rosuvastatin 40 mg) can reduce LDL-C by a maximum of 55%. Thus, in particular subjects with high baseline levels of LDL-C above 160 mg/dl will not achieve target which, based on most recent guidelines, is 70 mg/dl. The addition of other treatment modalities has several problems, either the yet unproven clinical efficacy of ezetimibe or a number of side effects with other compounds that prevent patients from taking this medication long-term like with bile acid sequestrants or nicotinic acid. Thus, there is still the need for further drugs to more potently lower LDL-C and get patients to target. In 2003, Abifadel et al. have described two families with autosomal dominant hypercholesterolemia which was associated with gain-of-function mutations in proprotein convertase s ubtilisin/kexin 9 ( PCSK9). This initial human experience was shortly followed by animal studies that identified a role for PCSK9 in the posttranslational regulation of the LDL receptor activity. PCSK9 is mainly synthesized in the liver. In the plasma where it binds to LDL receptors it reduces recycling, effectively down-regulating LDL receptor activity and results in increased plasma LDL-C levels. It is known that humans with gain-of-function mutations have higher plasma LDL-C and increased coronary heart disease risk while those with loss-of-function mutations have lower plasma LDL-C and reduced coronary artery disease risk. Thus, PCSK 9 may truly represent an exciting new target for treatment of hypercholesterolemia. Meanwhile several inhibitors of PCSK9 have been developed, either as fully humanized monoclonal antibodies, or using antisense technology or considering small molecules. Initial dosefinding studies have yielded promising results with maximum reduction of LDL-C on top of statin treatment in the order of 60-70%. So far, there are no major safety concerns but still a number of issues related to the long-term efficacy and also potential side-effects that need to be addressed in large phase III clinical trials. Once the efficacy in terms of a further reduction of cardiovascular events has been proven, these compounds will provide an additional therapeutic opportunity primarily in patients with homozygous and heterozygous familial hypercholesterolemia, but also for those with proven statin intolerance. Based on the resulting cost issues, further high-risk subgroups that would greatly benefit from this new therapy have to be carefully defined.
L’auteur n’a pas transmis de conflit d’intérêt concernant les données diffusées dans cette vidéo ou publiées dans la référence citée.
Conférence enregistrée : 9th Global Cardiovascular Clinical Trialists Forum • Paris 2012
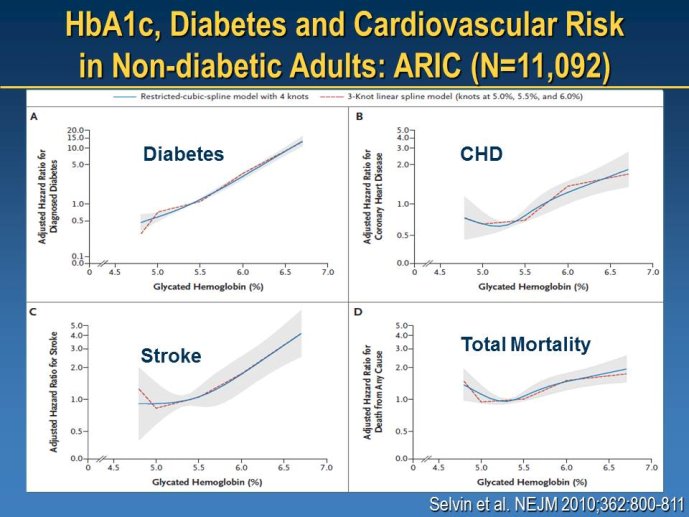
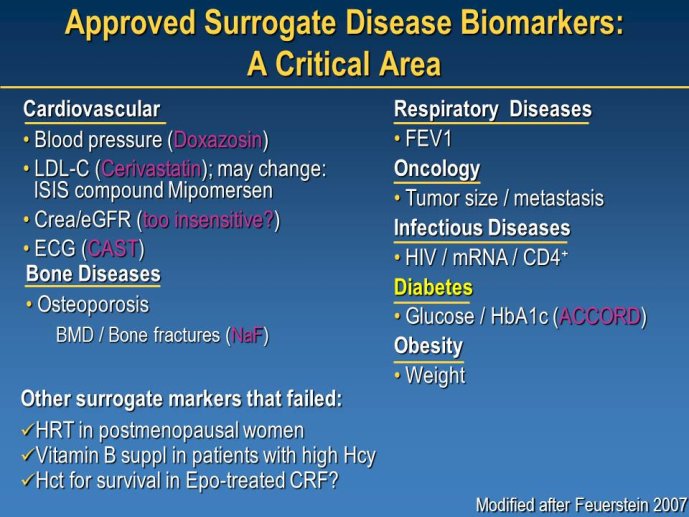
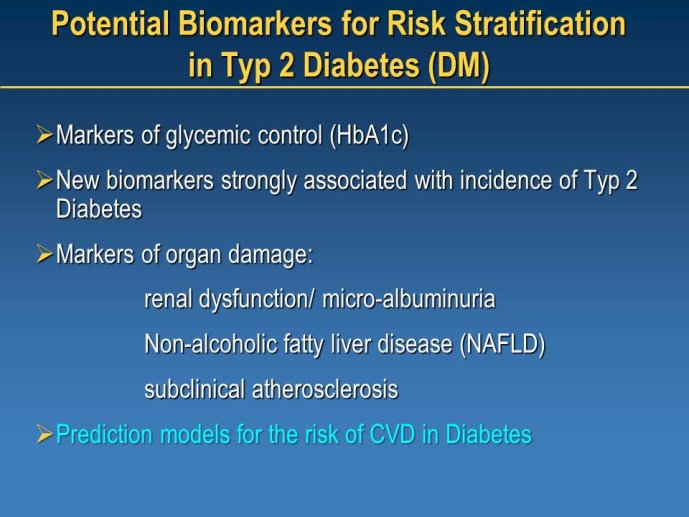
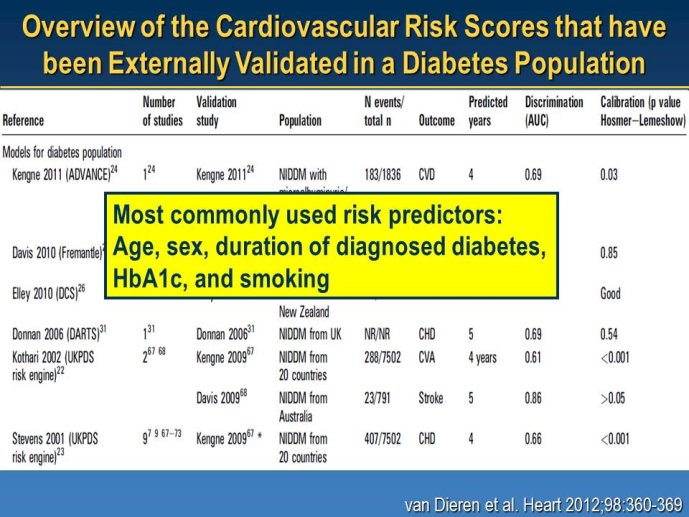
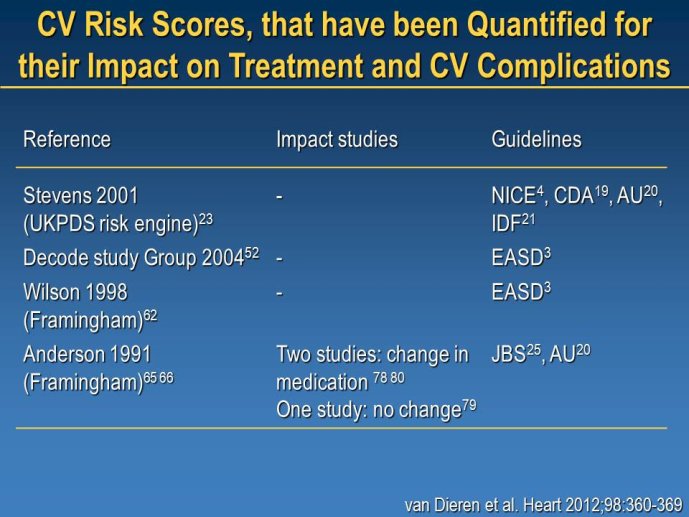
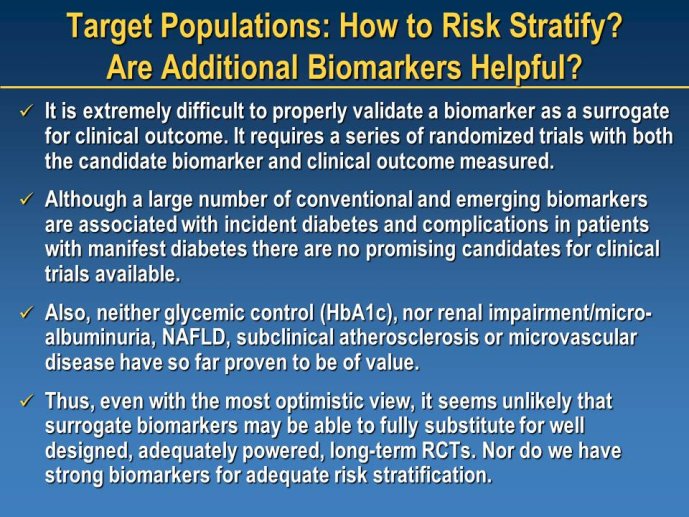
Diabetes clinical trials : helped or hindered by the current shift in regulatory requirements? Glycaemic control is an inadequate surrogate marker of cardiovascular event reduction in patients with type 2 diabetes. Clinical trials to date have been unsuccessful in identifying a therapeutic approach that addresses the underlying problem in diabetes (glycaemic control) and reduces cardiovascular risk. The potential for some agents to increase the risk of cardiovascular events has led to substantial changes in regulatory requirements for new anti-diabetic therapies. These requirements, while key to ensuring the cardiovascular safety of new agents, fail to emphasize the need to show clinical benefits, such as less visual impairment, less need for dialysis, or fewer cardiovascular events and deaths. Changes in test results such as glycaemic control, serum creatinine, micro-albuminuria, or retinopathy are inadequate surrogates. Regulators should consider the potential advantages of offering extended patent protection in order to encourage companies to conduct long-term trials in diabetes and many other chronic medical conditions. Cooperative efforts among physicians, clinical trialists, regulators, and sponsors are needed to address unresolved issues including re-defining therapeutic targets that are meaningful to patients with diabetes, determining the appropriate length of follow-up for future trials, and considering the ethical and operational challenges of non-inferiority designs.
Chairpersons: Marc PFEFFER, Boston, USA - Kausik RAY, London, GBR
Réalisation, production : Canal U/3S et CERIMES
Keyword : Cardiovascular Clinical Trialists, Paris, 2012, Cardiovascular prevention, Baro-stimulation, diabetes
Dans la même collection
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Non randomized and/…
POCOCK Stuart J.
MODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
ZANNAD Faiez
MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : How to secure the optima…
GIBSON Michael
MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : Industry viewpoint (Joer…
KOECK Jean-Louis
MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
DELIARGYRIS Efthymios
MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Industry perspectiv…
WOEHRLE Holger
MODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
PRASAD Krishna
MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : Different doses, differe…
VERHEUGT Freek
MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Options of and alte…
ABRAHAM William T.
MODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : How to secure the optima…
GELLER Nancy L.
MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 2 : Well Established Methods…
KOENIG Wolfgang
MODIGLIANI Workshop 2 - Friday November 30, 2012 : ATHEROSCLEROSIS IMAGING IN CLINICAL TRIALS Facilitating the discovery of effective therapies Chairpersons: Jagat NARULA, New York, USA - Ahmed
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Debate Session 5 : The Vaptans story …
ABRAHAM William T.
MODIGLIANI Debate Session 5 - Saturday December 1st, 2012 NOVEL DIURETIC STRATEGIES IN HEART FAILURE Chairpersons: Keld KJELDSEN, Copenhagen, DEN - Gian Paolo ROSSI, Padua, ITA Webcast: Patrick
Avec les mêmes intervenants et intervenantes
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 2 : Well Established Methods…
KOENIG Wolfgang
MODIGLIANI Workshop 2 - Friday November 30, 2012 : ATHEROSCLEROSIS IMAGING IN CLINICAL TRIALS Facilitating the discovery of effective therapies Chairpersons: Jagat NARULA, New York, USA - Ahmed
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 5 : Identifying new targets:…
KOENIG Wolfgang
Will new compounds be able to reduce the residual risk in high risk patients when treatment targets based on new ESC guidelines have been achieved (e.g. LDL-C lesser than 70mg/dl)?