Notice
Cardiovascular Clinical Trialists (CVCT) Forum - Paris 2012 : Globalization of Diabetes trials: Epidemiology of diabetes in the Middle East and Asian countries
- document 1 document 2 document 3
- niveau 1 niveau 2 niveau 3
Descriptif
Title : Globalization of Diabetes trials: Epidemiology of diabetes in the Middle East and Asian countries
Speaker: Prem PAIS, Bangalore, IND
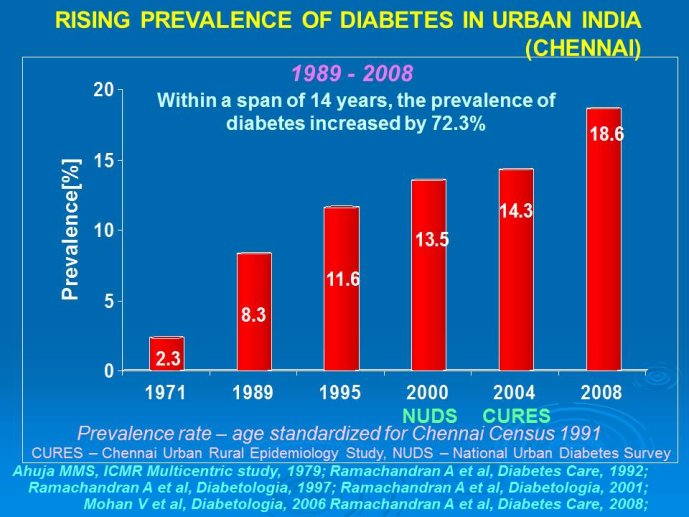
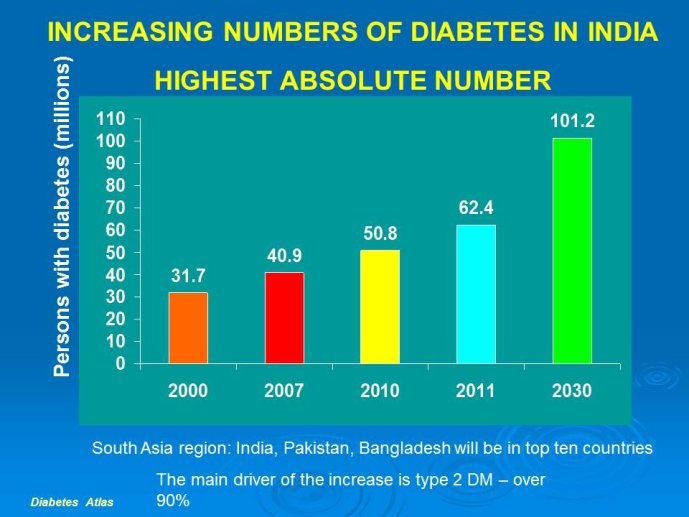
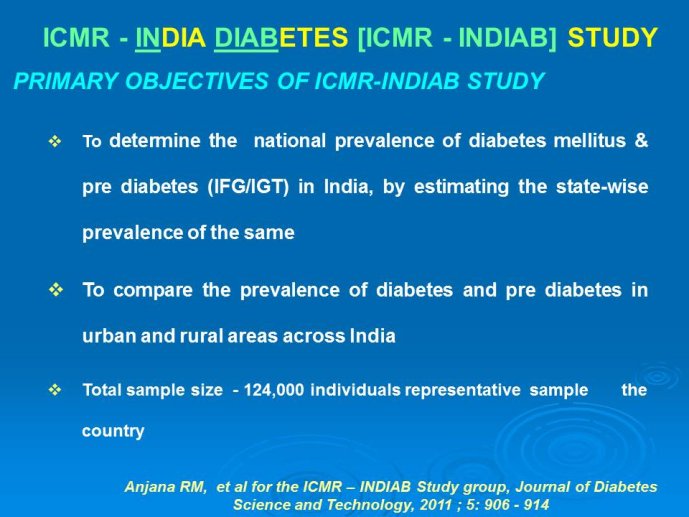
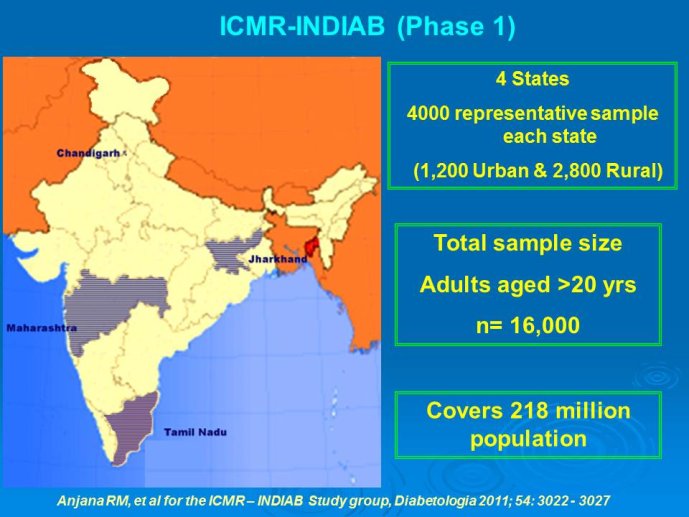
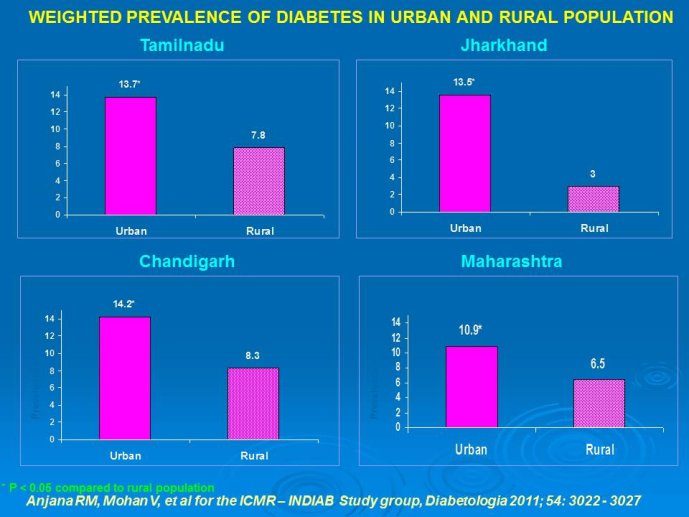
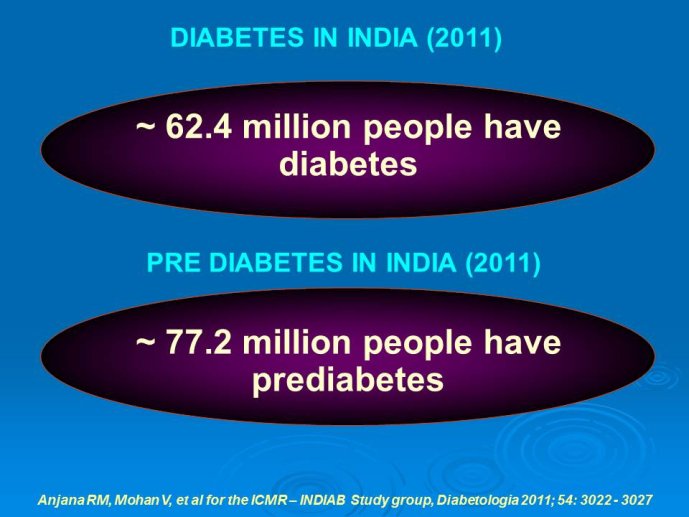
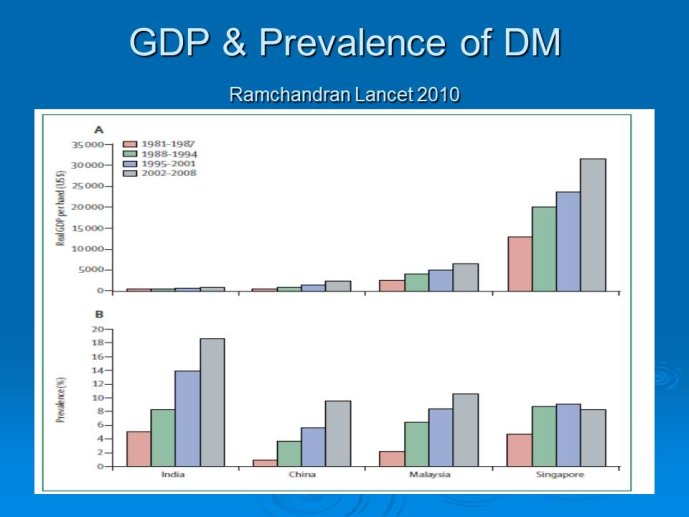
Abstract : India has a high prevalence of type 2 diabetes mellitus (DM) and the highest absolute number of diabetic people in the world – 50 million in 2006, estimated to increase to70 million by 2025. While there is no representative national survey, cross sectional studies in different parts of the country have demonstrated an increasing prevalence in both rural and urban areas of the country. An early survey done between 1972 -1975 on 35 000 subjects aged over 14 years reported a prevalence of DM of 2.1% in urban and 1.5% in rural regions; in those aged over 40 years the prevalence was 5.0% and 2.8 % respectively. By 2010 a survey in adults aged over 20 years showed the prevalence to be as high as 13% and 7.8% in urban and rural areas respectively in the state of Tamil Nadu. Prediabetes – IGT and IFG – was an additional 9.8% (urban) and 7.1% (rural). In addition there is a high prevalence of undiagnosed disease.
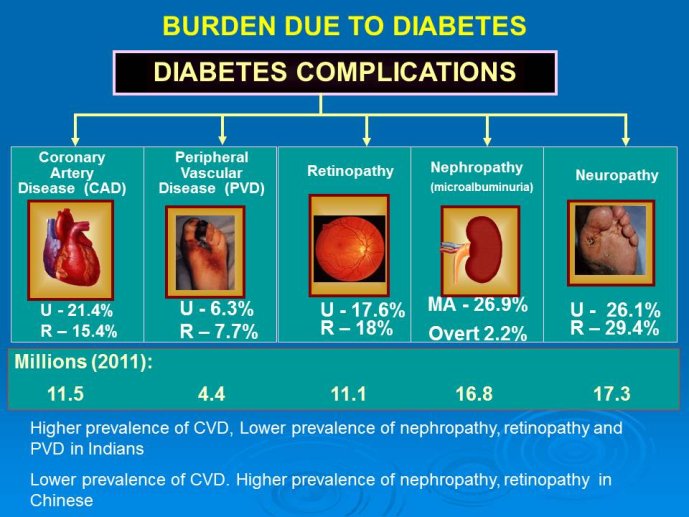
Burden of complications: Indians with DM are at high risk of CVD especially CAD while risk of PVD seems to be less than in Western populations. In the CUPS study in Tamil Nadu, South India, the prevalence of CAD in diabetics was 21.4% compared to 9.1% in age matched non diabetic controls while the prevalence of PVD was 6.3% and 2.7% in diabetics and nondiabetics respectively. As regards microvascular complications, the prevalence is lower than is reported in other populations including the Chinese population. Overall retinopathy prevalence is 17.6%, microalbuminuria 26.9% and over nephropathy 2.2%.
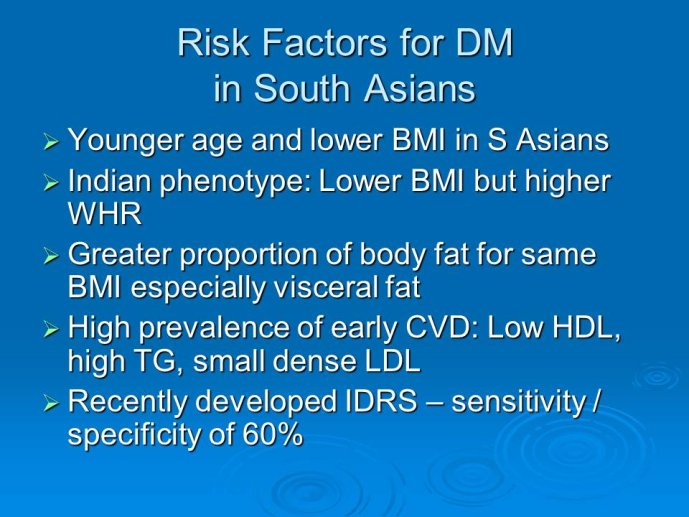
Risk factors for DM. Risk factors for DM are no different from other populations – obesity, sedenteryness and a family predilection. However risks seem to rise at younger age and at lower BMI than in Caucasians. In addition for a given BMI,
Indians have higher total abdominal and visceral fat. The genetic determinants of this are yet to be fully understood.
Risk score. To aid in population screening for DM a nonlaboratory based risk scoring system has been developed and validated – the Indian Diabetes Risk Score (IDRS) with a sensitivity and specificity of over 60%.
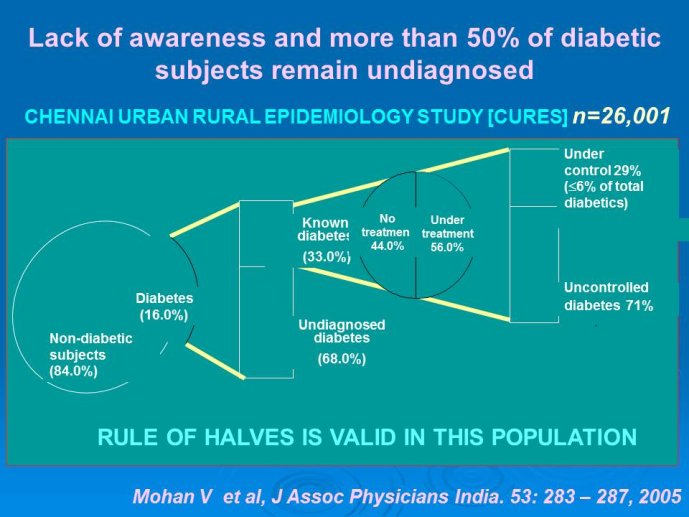
Management status. Appropriate management and diagnosis of DM is unsatisfactory. In one survey in an urban South India, 68% of detected cases were previously undiagnosed. Of diagnosed patients, only 56% were on treatment and of these 29% had satisfactory control.
Regulatory Issues. Regulatory approval and monitoring of clinical trials in India are currently in a state of flux after an intense and often uninformed media focus. As new systems and regulations are being developed and enforced, approvals for new studies may take six months after submission. With specific reference to trials in DM, studies in subjects aged over 65 years require specific justification for their inclusion as such people are being viewed as a vulnerable population. Recently approval for a long term trial for cardiovascular safety of dulaglutide was denied because the product had not yet been approved for it hypoglycemic effect. The sponsor has reapplied for approval and is awaiting the response.
L’auteur n’a pas transmis de conflit d’intérêt concernant les données diffusées dans cette vidéo ou publiées dans la référence citée.
Conférence enregistrée : 9th Global Cardiovascular Clinical Trialists Forum • Paris 2012
Diabetes clinical trials : helped or hindered by the current shift in regulatory requirements? Glycaemic control is an inadequate surrogate marker of cardiovascular event reduction in patients with type 2 diabetes. Clinical trials to date have been unsuccessful in identifying a therapeutic approach that addresses the underlying problem in diabetes (glycaemic control) and reduces cardiovascular risk. The potential for some agents to increase the risk of cardiovascular events has led to substantial changes in regulatory requirements for new anti-diabetic therapies. These requirements, while key to ensuring the cardiovascular safety of new agents, fail to emphasize the need to show clinical benefits, such as less visual impairment, less need for dialysis, or fewer cardiovascular events and deaths. Changes in test results such as glycaemic control, serum creatinine, micro-albuminuria, or retinopathy are inadequate surrogates. Regulators should consider the potential advantages of offering extended patent protection in order to encourage companies to conduct long-term trials in diabetes and many other chronic medical conditions. Cooperative efforts among physicians, clinical trialists, regulators, and sponsors are needed to address unresolved issues including re-defining therapeutic targets that are meaningful to patients with diabetes, determining the appropriate length of follow-up for future trials, and considering the ethical and operational challenges of non-inferiority designs.
Chairpersons: Marc PFEFFER, Boston, USA - Kausik RAY, London, GBR
Réalisation, production : Canal U/3S et CERIMES
Keyword : Cardiovascular Clinical Trialists, Paris, 2012, Cardiovascular prevention, Baro-stimulation, diabetes
Intervention / Responsable scientifique
Dans la même collection
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
DeliargyrisEfthymiosMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Industry perspectiv…
WoehrleHolgerMODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
PrasadKrishnaMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : Different doses, differe…
VerheugtFreekMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Non randomized and/…
PocockStuart J.MODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
ZannadFaiezMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : How to secure the optima…
GibsonMichaelMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : Industry viewpoint (Joer…
KoeckJean-LouisMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 2 : Well Established Methods…
KoenigWolfgangMODIGLIANI Workshop 2 - Friday November 30, 2012 : ATHEROSCLEROSIS IMAGING IN CLINICAL TRIALS Facilitating the discovery of effective therapies Chairpersons: Jagat NARULA, New York, USA - Ahmed
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Options of and alte…
AbrahamWilliam T.MODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : How to secure the optima…
GellerNancy L.MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Debate Session 5 : Ultrafiltration fo…
RossiGian PaoloMODIGLIANI Debate Session 5 - Saturday December 1st, 2012 NOVEL DIURETIC STRATEGIES IN HEART FAILURE Chairpersons: Keld KJELDSEN, Copenhagen, DEN - Gian Paolo ROSSI, Padua, ITA Webcast: Patrick