Notice
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 : Biomarker guided-therapy: Are there other endpoints besides mortality that matter? Selecting the best outcome measures
- document 1 document 2 document 3
- niveau 1 niveau 2 niveau 3
Descriptif
Title : Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 : Biomarker guided-therapy: Are there other endpoints besides mortality that matter? Selecting the best outcome measures
Speaker: Michael FELKER, Durham, USA
Abstract : Biomarker guided-therapy: Are there other endpoints besides mortality that matter? Selecting the best outcome measures
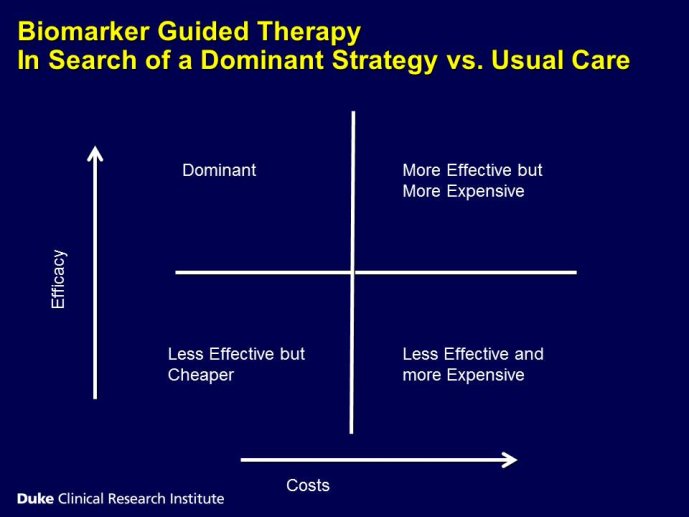
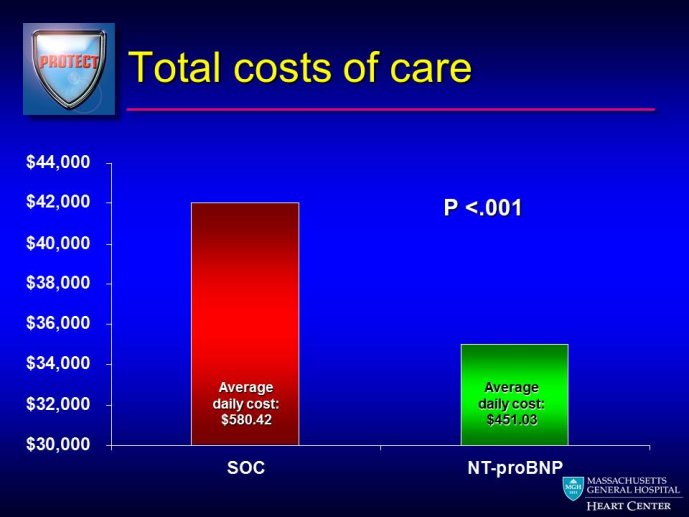
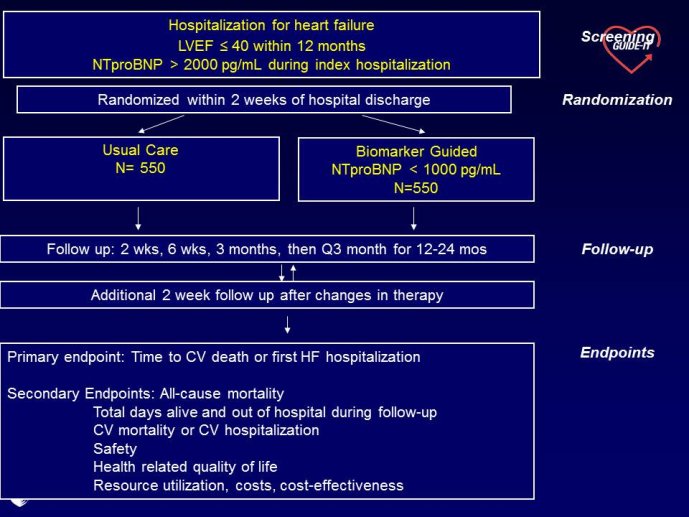
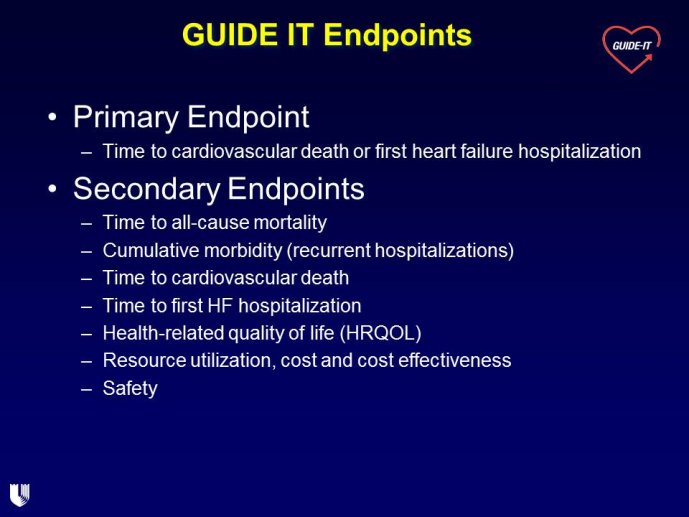
The measurement of biomarkers for diagnosis and prognosis in heart disease has changed the fundamental way that patients are evaluated, and has led to a literal explosion of studies exploring both novel applications of established biomarkers as well as the discovery of newer biological markers. With the rise in interest in novel biomarkers has come a clear heterogeneity in the approach with which these potentially important tests have been studied, partially due to a lack of guidance as to nominal expectations for the approach for their evaluation. To this point, there is no consensus yet articulated with respect to the minimum expectations for which a new diagnostic or prognostic biomarker should be held to in order to clarify or reject their potential value. We recently proposed several standards to which novel biomarkers in heart failure should be held during their evaluation (Table) (1); these “rules” do not specifically apply to heart failure per se, and could thus theoretically serve as a starting point for determining the basic framework upon which the evaluation of novel applications of prior markers or the description of a frankly new biomarker.
1. The method by which a novel biomarker is judged (including and especially when compared to or in combination with other biomarkers) should be thorough: novel tests should be evaluated across a wide range of patients typical of the diagnosis for which it will be applied, and the statistical methods used to evaluate the biomarker (relative to clinical variables as well as other biomarkers) should be contemporary, rigorous, standardized and fair.
2. Measurement of a novel HF biomarker (e.g. in blood, urine or any easy obtainable tissue) should be easily achieved within a short period of time, provide acceptable accuracy and assays for its measurement should have defined biological variation and low analytical imprecision.
3. The biomarker should primarily reflect important (patho) physiological process(es) involved in HF presence and progression; use of biomarkers reflective of disease but originating outside the myocardium is acceptable as long as such a biomarker provides independently useful information involved in the diagnosis, prognosis, progression or therapy of HF syndromes.
4. The biomarker must provide clinically useful information for caregivers (physician, nurses, patient and others) to more swiftly and reliably establish/reject a diagnosis, to more accurately estimate prognosis, or to inform more successful therapeutic strategies. The information from such a biomarker should not recapitulate clinical information already available at the bedside, and must be additional to other biomarkers.
L’auteur n’a pas transmis de conflit d’intérêt concernant les données diffusées dans cette vidéo ou publiées dans la référence citée.
9th Global Cardiovascular Clinical Trialists Forum • Paris 2012
WHAT IS THE OPTIMAL DESIGN FOR BIOMARKER STUDIES?
Chairpersons: Jim JANUZZI, Boston, USA - Faiez ZANNAD, Nancy, FRA
Réalisation, production : Canal U/3S et CERIMES
Keyword : Cardiovascular Clinical Trialists, Paris, 2012, Cardiovascular prevention, cardiovascular pharmacology, biomarker
Intervention / Responsable scientifique
Dans la même collection
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
DeliargyrisEfthymiosMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Industry perspectiv…
WoehrleHolgerMODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
PrasadKrishnaMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : Different doses, differe…
VerheugtFreekMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Non randomized and/…
PocockStuart J.MODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
ZannadFaiezMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : How to secure the optima…
GibsonMichaelMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : Industry viewpoint (Joer…
KoeckJean-LouisMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 2 : Well Established Methods…
KoenigWolfgangMODIGLIANI Workshop 2 - Friday November 30, 2012 : ATHEROSCLEROSIS IMAGING IN CLINICAL TRIALS Facilitating the discovery of effective therapies Chairpersons: Jagat NARULA, New York, USA - Ahmed
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Options of and alte…
AbrahamWilliam T.MODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : How to secure the optima…
GellerNancy L.MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Debate Session 5 : Ultrafiltration fo…
RossiGian PaoloMODIGLIANI Debate Session 5 - Saturday December 1st, 2012 NOVEL DIURETIC STRATEGIES IN HEART FAILURE Chairpersons: Keld KJELDSEN, Copenhagen, DEN - Gian Paolo ROSSI, Padua, ITA Webcast: Patrick
Avec les mêmes intervenants et intervenantes
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Debate Session 5 : The Vaptans story …
FelkerG. MichaelMODIGLIANI Debate Session 5 - Saturday December 1st, 2012 NOVEL DIURETIC STRATEGIES IN HEART FAILURE Chairpersons: Keld KJELDSEN, Copenhagen, DEN - Gian Paolo ROSSI, Padua, ITA Webcast: Patrick