Notice
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 3 : ASTRONAUT (Aldo MAGGIONI)
- document 1 document 2 document 3
- niveau 1 niveau 2 niveau 3
Descriptif
MODIGLIANI Lunch Debate Session 3 - Saturday December 1st, 2012:
HEART FAILURE TRIALISTS WORKSHOP
LEARNING FROM RECENT TRIALS AND SHAPING THE FUTURE OF HEART FAILURE TRIALS
Chairpersons: Alexandre MEBAZAA, Paris, FRA - Christopher O’CONNOR, Durham, USA
Webcast: Daniela DOBRE, Nancy, FRA
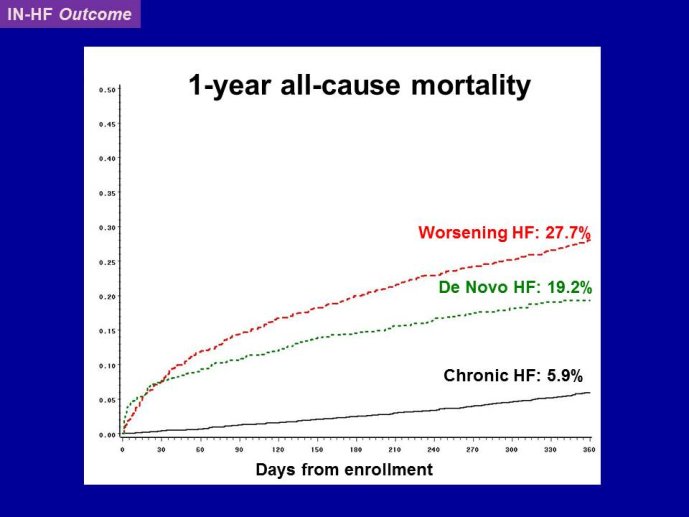
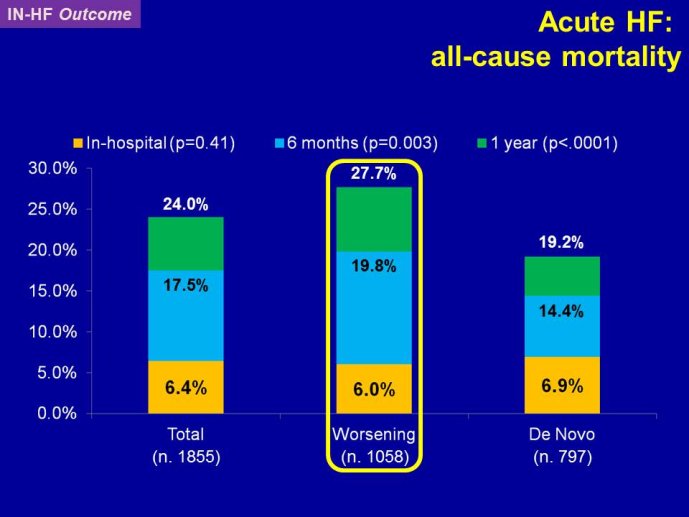
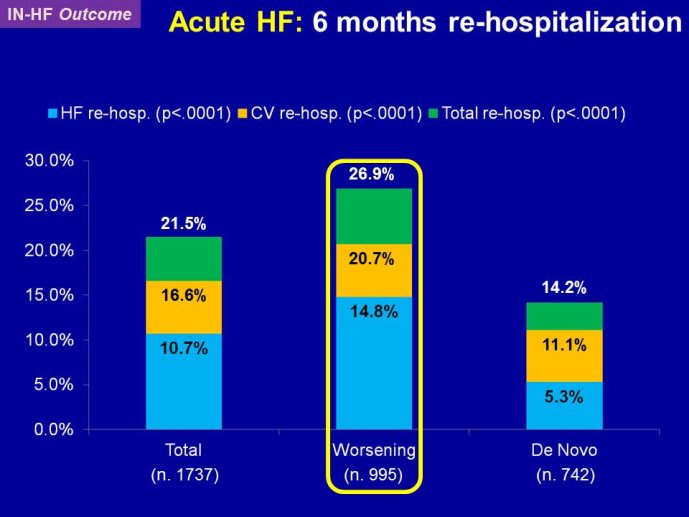
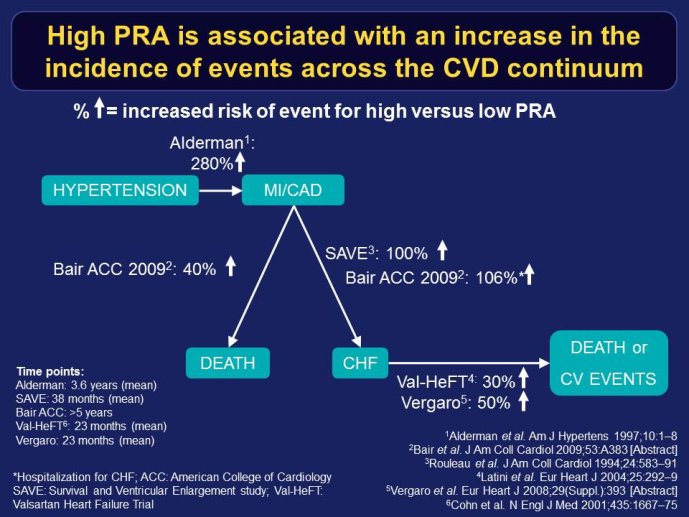
Decreasing the very high mortality and rate of re-hospitalization associated with acute worsening HF is one of the most important unmet needs in cardiovascular medicine. Despite positive signals in Phase II studies, no drug has proven to reduce the appallingly high mortality or readmission rates. The reasons for these ‘failed’ trials are multiple, including the adequacy of candidate drugs, dosing, patient selection and disease characterization, and trial conduct. The phenotypes and pathophysiology of the syndrome are poorly understood. The syndrome is heterogeneous and the taxonomy is complex and remains without a consensus. Hospitalization for acute worsening HF is understood by some as the result of progressive worsening of chronic HF and by others as an entity which acuteness has been compared to what is acute coronary syndrome to chronic coronary artery disease. Some believe that hospitalizations for HF do not represent a distinct pathophysiology than chronic HF and could be better managed by the optimization of chronic HF neurohumoral therapy. On the other hand although there are no data to support that short-term in-patient therapeutic approach improves post-discharge clinical outcomes, some speculate that protecting the injured heart during the acute process might have long term benefit. Alleviating dyspnea is still considered by regulatory agents as an endpoint valid in itself, as far as there is no excess of deaths. However most patients improve with standard therapy and the magnitude of additional dyspnea relief by the investigational drug might be marginal and therefore hardly statistically detectable. Alternative ways to test for and/or quantify dyspnea have been investigated with the aim to define a dyspnea measure that is more sensitive to change.
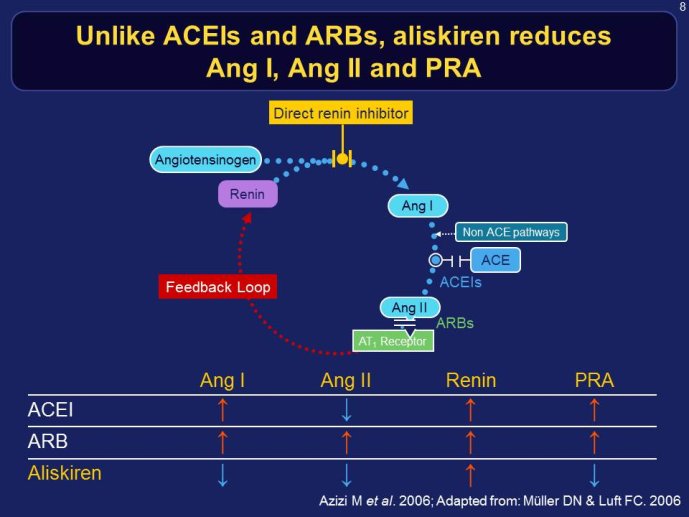
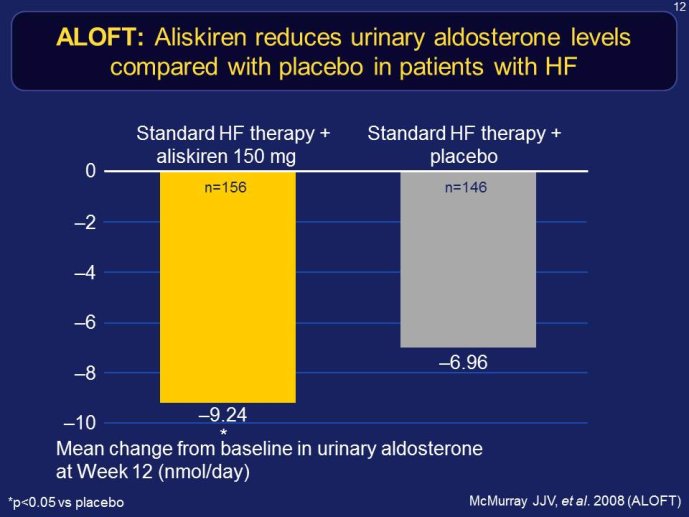
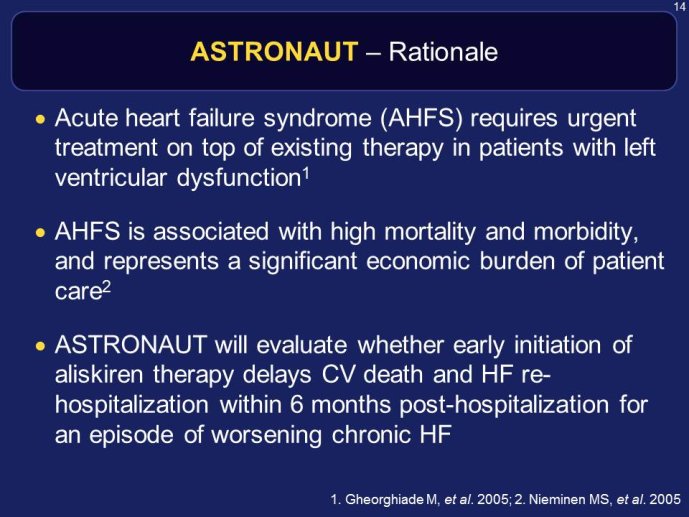
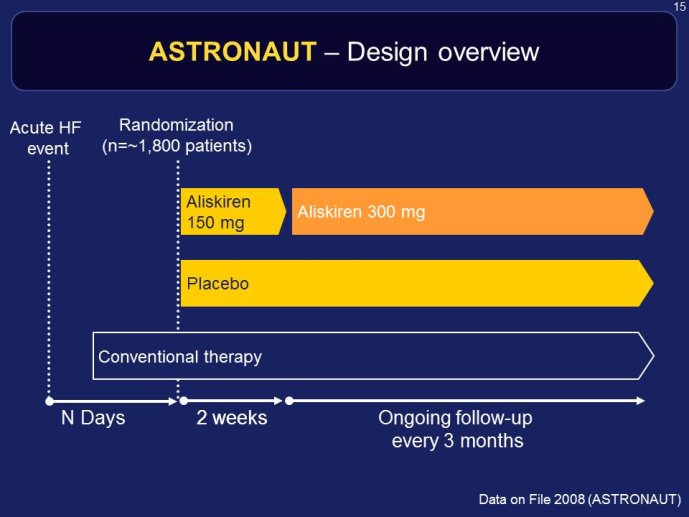
RELAX-HF and ASTRONAUT are two trials whose results have just been announced. Both exemplify the issues highlighted here above. RELAX-HF is a phase II/III trial design trial of 48 hour IV infusion of relaxin for the treatment of signs and symptoms in patients hospitalized for acute decompensated HF (no EF criteria). ASTRONAUT evaluates the 6 months efficacy and safety of aliskiren therapy on top of standard therapy, on morbidity and mortality when initiated early after
hospitalization for acute decompensated HF and low EF. Results of PRONTO are now available. PRONTO is a randomized trial comparing the potent and rapid acting calcium channel blocker Clevidipine vs. SOC for the ability to rapidly control blood pressure and provide dyspnea relief in acute heart failure patients. PRONTO examplifies yet another trial model: intervening within the first 2 hours after admission. Lessons learnt from RELAX-HF and PRONTO within the context of other recent trials in acute heart failure will be the main topic of brainstorming at this workshop, examining the potential change in paradigm in this area.
The aim of this workshop is to learn from the PRONTO, RELAX-HF and ASTRONAUT experiences within the context of the other acute HF trials, understand the consequences of the results on the design of future trials, revision of regulatory guidlenes and on possible regulatory labeling on clinical practice.
The various drug intervention options
RELAX-HF, Omecamtiv mecarbil and other drug still on trial
Speaker: Michael FELKER, Durham, USA
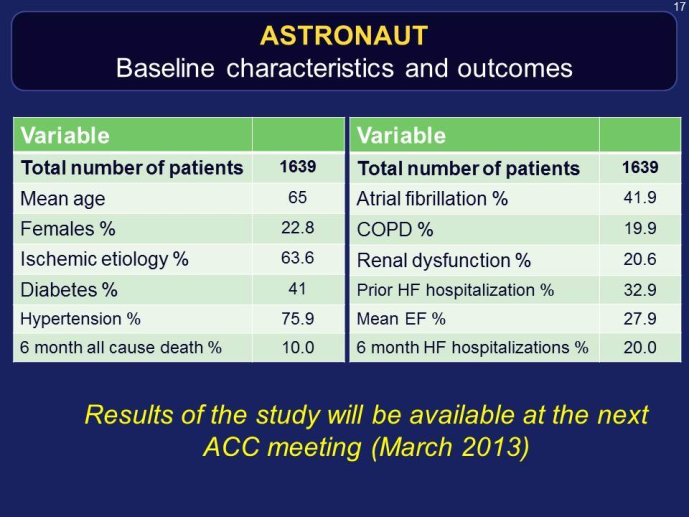
ASTRONAUT
Speaker: Aldo MAGGIONI, Florence, ITA
Execution issues. Where best to screen and enroll patients? Overcoming variations in health care systems and globalization issues
Speaker: Mihai GHEORGHIADE, Chicago, USA
PRONTO: The merit and consequences of a very early intervention with an arterial vasodilator
Speaker: Frank PEACOCK, Cleveland, USA
Endpoint related issues. The value of dyspnea as an endpoint in acute HF, upon admission trials
Speaker: Alexandre MEBAZAA, Paris, FRA
The value of repeat events in post discharge hospitalized HF trials
Speaker: Stuart POCOCK, London, GBR
Regulatory viewpoint: Yuki ANDO, PMDA, JAP - Robert HEMMINGS, MHRA, GBR
Thème
Documentation
Liens
Dans la même collection
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
ZANNAD Faiez
MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : How to secure the optima…
GIBSON Michael
MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : Industry viewpoint (Joer…
KOECK Jean-Louis
MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
DELIARGYRIS Efthymios
MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Industry perspectiv…
WOEHRLE Holger
MODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
PRASAD Krishna
MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : Different doses, differe…
VERHEUGT Freek
MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Non randomized and/…
POCOCK Stuart J.
MODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : How to secure the optima…
GELLER Nancy L.
MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 2 : Well Established Methods…
KOENIG Wolfgang
MODIGLIANI Workshop 2 - Friday November 30, 2012 : ATHEROSCLEROSIS IMAGING IN CLINICAL TRIALS Facilitating the discovery of effective therapies Chairpersons: Jagat NARULA, New York, USA - Ahmed
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Options of and alte…
ABRAHAM William T.
MODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Debate Session 5 : The Vaptans story …
ABRAHAM William T.
MODIGLIANI Debate Session 5 - Saturday December 1st, 2012 NOVEL DIURETIC STRATEGIES IN HEART FAILURE Chairpersons: Keld KJELDSEN, Copenhagen, DEN - Gian Paolo ROSSI, Padua, ITA Webcast: Patrick
Sur le même thème
-
La naissance de la médecine scientifique (par Pierre Corvol)
CORVOL Pierre
MONTENOT Jean
La naissance de la médecine scientifique Dans La Maison Nuncingen (1837), Balzac met en scène une conversation entre quatre journalistes échauffés par un bon repas. L’un des commensaux, Émile
-
Prévention de l'accident vasculaire cérébral
Chaque année, en France, près de 125 000 cas d'AVC -- Accident Vasculaire Cérébral -- sont recensés. Avec les récidives, ce chiffre augmente de 25 % L' AVC représente la 1ère cause de handicap et
-
Michel Haïssaguerre, entre rythmes et musicalité
GLOINEC Yves
PRE Nadège
A l’occasion de la création de l’Institut Hospitalo-Universitaire LIRYC (Institut de Rythmologie et de Modélisation Cardiaque) dont il est à l’origine, le 2e volet de notre série Trip TIC
-
Du coté de chez...Michel Haïssaguerre
GLOINEC Yves
PRE Nadège
Inspiré du questionnaire de Proust, cet entretien plus intimiste engage une réflexion sur Michel Haïssaguerre en tant qu’homme et non plus en tant que cardiologue. Ce face-à-face permet de
-
Cardio-vasculaire
GAY Bernard
GOSSE Philippe
DOUARD Hervé
SASSOUST Gérard
Journées Bordeaux Segalen 2013 - Formation Médicale Continue des Médecins Généralistes - Session Cardio-vasculaire
-
Valvulopathies du cœur droit
LAFITTE Stéphane
COTTARRE-LAFITTE Marianne
RéANT Patricia
ROUDAUT Raymond
Ce cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,
-
Échographie du sujet âgé
LAFITTE Stéphane
COTTARRE-LAFITTE Marianne
RéANT Patricia
ROUDAUT Raymond
Ce cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,
-
Myocardiopathies hypertrophiques
LAFITTE Stéphane
COTTARRE-LAFITTE Marianne
RéANT Patricia
ROUDAUT Raymond
Ce cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,
-
Segmentation cardiaque
LAFITTE Stéphane
COTTARRE-LAFITTE Marianne
RéANT Patricia
ROUDAUT Raymond
Ce cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,
-
Doppler tissulaire en pratique ECG
LAFITTE Stéphane
COTTARRE-LAFITTE Marianne
RéANT Patricia
ROUDAUT Raymond
Ce cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,
-
Insuffisance cardiaque et pronostic
LAFITTE Stéphane
COTTARRE-LAFITTE Marianne
RéANT Patricia
ROUDAUT Raymond
Ce cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,
-
La péricardite liquidienne
LAFITTE Stéphane
COTTARRE-LAFITTE Marianne
RéANT Patricia
ROUDAUT Raymond
Ce cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,