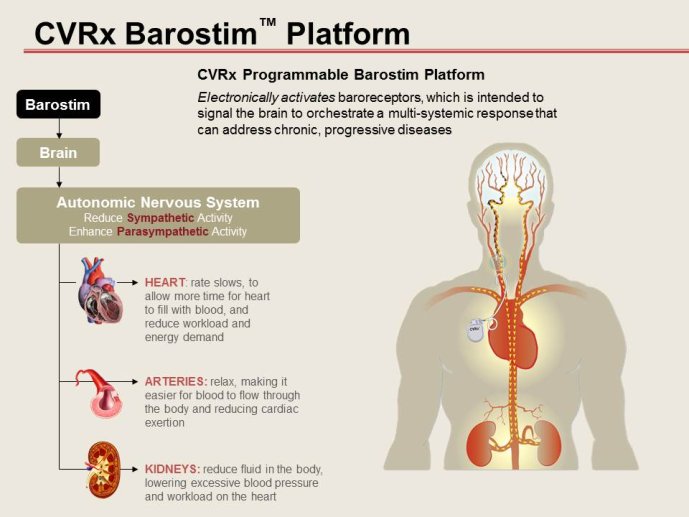
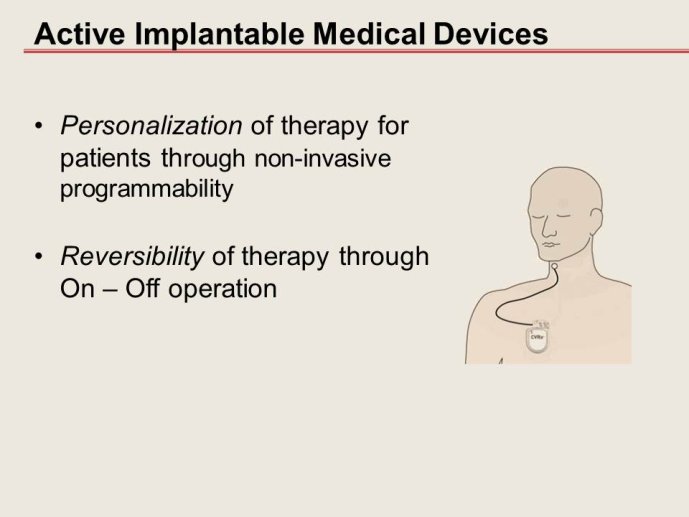
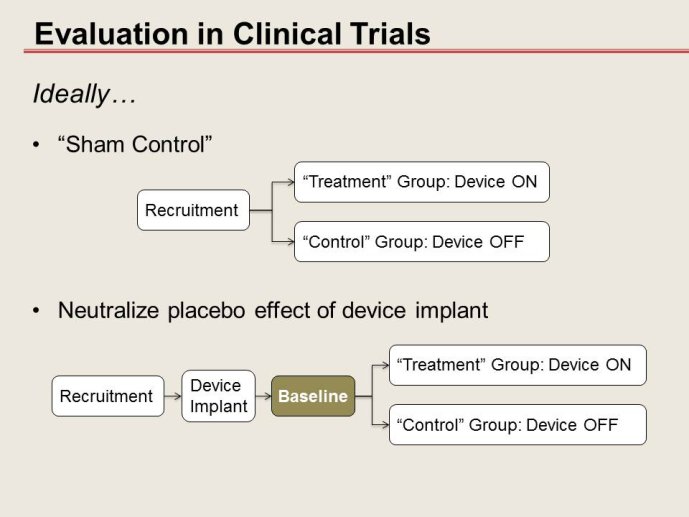
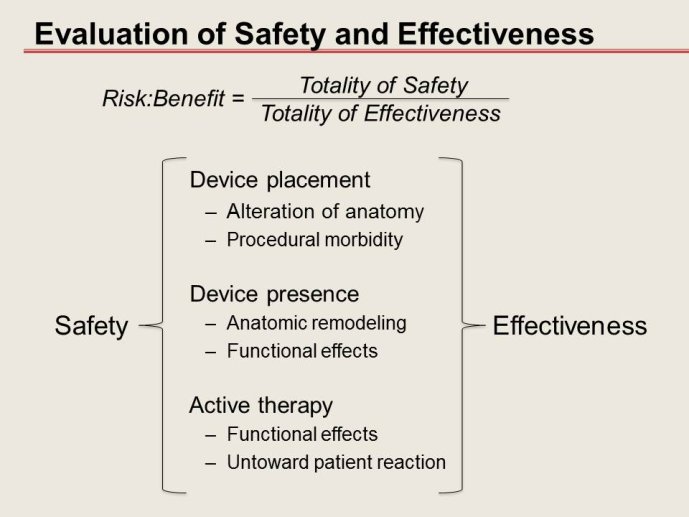
Notice
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Industry perspective (Rob KIEVAL)
- document 1 document 2 document 3
- niveau 1 niveau 2 niveau 3
Descriptif
MODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012
THE DEVICE THERAPY TRIALISTS WORKSHOP
Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA
Webcast: Tariq AHMAD, Durham, USA
Device trial methodology, regulatory and implementation issues
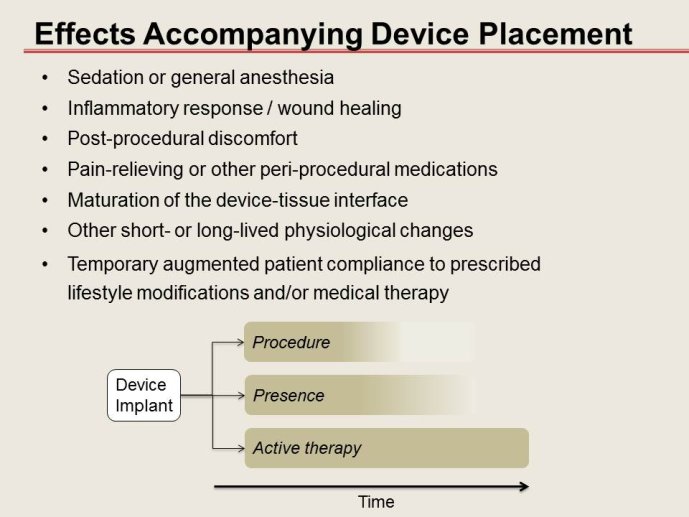
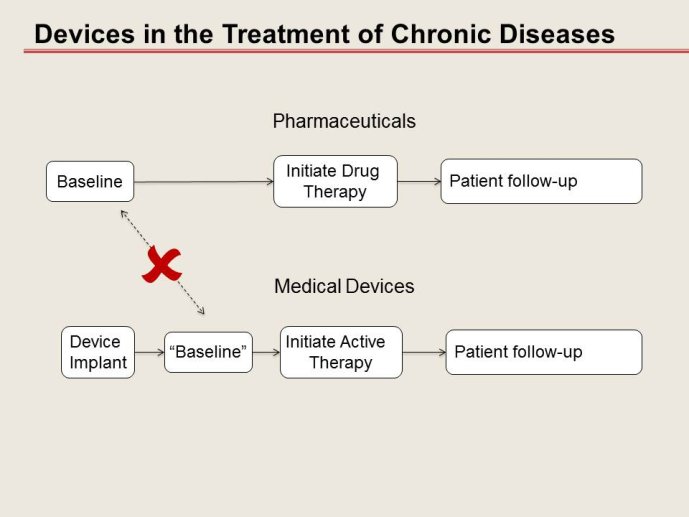
Advances in interventional medical devices are increasingly affecting cardiovascular therapy, just as pharmacological innovation did the generation before. Yet, designing and conducting a device trial is challenging and drug trial designs may not necessarily be applied fully to device trials.
➢ Although there is increasing recognition that this development process substantially differs from that for drugs, how much device trial methodology may deviate from drug trial methodology is a matter of discussion.
➢ Regulation works differently for drugs, devices, and procedures, and there are wide international variations. Although progressively moving toward some alignment, currently, in Europe, industry needs only to fulfill the (light) criteria of “CE” mark before approval.
General sale of devices may be permitted on the basis of proof of safety, rather than of efficacy or effectiveness. In any case, reimbursement claims may require collecting evidence in outcome cost-effectiveness trials. Regulators and commercial bodies should seek consensus.
➢ Innovation is led by device industry which is facing the economic challenge of bringing innovation to the market in a very competitive environment. Multi sponsored trials and the cooperation with public funders may be instrumental in improving knowledge production for a better device therapy.
➢ Interpretation of trial results, and consequently therapy adoption, is another challenge. The strength of evidence is not necessarily the main driver for adoption. Coronary angioplasty in stable CAD is widely adopted while, despite evidence for a beneficial effect of device therapy in heart failure, only a minority of eligible patients is currently offered these options.
➢ Beyond trials aimed at evaluating safety, effectiveness and approval, trials that establish the value of a therapy and hence support utilization in clinical practice are most needed.
The aim of this session is to contribute to identifying and promoting innovative, cooperative and practical solutions that may help filling the gaps between device and drug trials, between CE mark and FDA regulations and between generating evidence and practical implementations.
Session program:
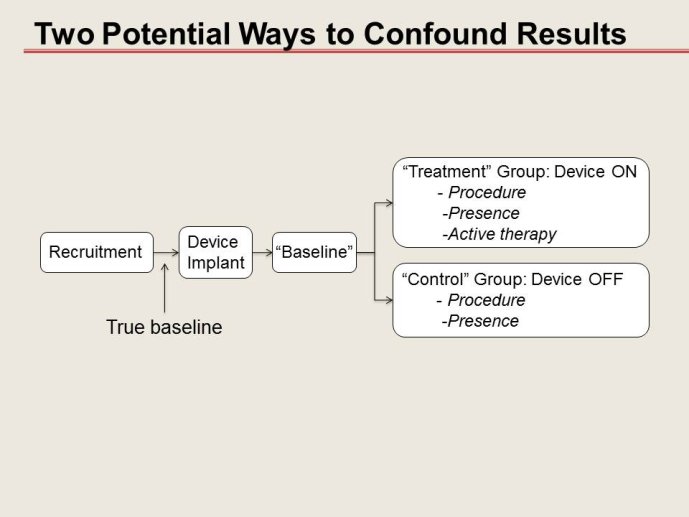
How much one could deviate from “randomized - controlled” trials?
• Non randomized and/or non-blinded trials: When can they be trusted, what can help them to be “acceptable”?
Speaker: Stuart POCOCK, London, GBR
• Options of and alternatives to the “control group” in device trials
Speaker: William T. ABRAHAM, Columbus, USA
• Industry perspective
Speakers: Rob KIEVAL, CVRx, USA - Holger WOEHRLE, ResMed, GER
Approvability issues: Pathway to a more global device approval process
Speaker: Ileana PIÑA, New York, USA
Post approval and registry studies. Advantages and limitations in complementing trial evidence base and improving therapy adoption
Speaker: Ileana PIÑA, New York, USA
Discussant: Roxana MEHRAN, New York, USA
Comparative effectiveness studies. How they may help decision makers and support utilization in clinical practice?
Speaker: Rita REDBERG, San Francisco, USA
Discussant: Kenneth STEIN, Boston Scientific, USA
Intervention / Responsable scientifique
Thème
Documentation
Liens
Dans la même collection
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : How to secure the optima…
GibsonMichaelMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : Industry viewpoint (Joer…
KoeckJean-LouisMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
DeliargyrisEfthymiosMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Industry perspectiv…
WoehrleHolgerMODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
PrasadKrishnaMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : Different doses, differe…
VerheugtFreekMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Non randomized and/…
PocockStuart J.MODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
ZannadFaiezMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : How to secure the optima…
GellerNancy L.MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 2 : Well Established Methods…
KoenigWolfgangMODIGLIANI Workshop 2 - Friday November 30, 2012 : ATHEROSCLEROSIS IMAGING IN CLINICAL TRIALS Facilitating the discovery of effective therapies Chairpersons: Jagat NARULA, New York, USA - Ahmed
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Options of and alte…
AbrahamWilliam T.MODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 5 : Will we be able to answe…
StroesEricWill new compounds be able to reduce the residual risk in high risk patients when treatment targets based on new ESC guidelines have been achieved (e.g. LDL-C lesser than 70mg/dl)?
Sur le même thème
-
La naissance de la médecine scientifique (par Pierre Corvol)
CorvolPierreMontenotJeanLa naissance de la médecine scientifique Dans La Maison Nuncingen (1837), Balzac met en scène une conversation entre quatre journalistes échauffés par un bon repas. L’un des commensaux, Émile
-
Prévention de l'accident vasculaire cérébral
Chaque année, en France, près de 125 000 cas d'AVC -- Accident Vasculaire Cérébral -- sont recensés. Avec les récidives, ce chiffre augmente de 25 % L' AVC représente la 1ère cause de handicap et
-
Michel Haïssaguerre, entre rythmes et musicalité
GloinecYvesPreNadègeA l’occasion de la création de l’Institut Hospitalo-Universitaire LIRYC (Institut de Rythmologie et de Modélisation Cardiaque) dont il est à l’origine, le 2e volet de notre série Trip TIC
-
Du coté de chez...Michel Haïssaguerre
GloinecYvesPreNadègeInspiré du questionnaire de Proust, cet entretien plus intimiste engage une réflexion sur Michel Haïssaguerre en tant qu’homme et non plus en tant que cardiologue. Ce face-à-face permet de
-
Cardio-vasculaire
GayBernardGossePhilippeDouardHervéSassoustGérardJournées Bordeaux Segalen 2013 - Formation Médicale Continue des Médecins Généralistes - Session Cardio-vasculaire
-
Doppler tissulaire en pratique ECG
LafitteStéphaneCottarre-LafitteMarianneRéantPatriciaRoudautRaymondCe cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,
-
Insuffisance cardiaque et pronostic
LafitteStéphaneCottarre-LafitteMarianneRéantPatriciaRoudautRaymondCe cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,
-
La péricardite liquidienne
LafitteStéphaneCottarre-LafitteMarianneRéantPatriciaRoudautRaymondCe cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,
-
La tamponnade
LafitteStéphaneCottarre-LafitteMarianneRéantPatriciaRoudautRaymondCe cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,
-
Échocardiographie trans-œsophagienne
LafitteStéphaneCottarre-LafitteMarianneRéantPatriciaRoudautRaymondCe cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,
-
Échocardiographie-Doppler normale
LafitteStéphaneCottarre-LafitteMarianneRéantPatriciaRoudautRaymondCe cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,
-
Indications de l'ETO
LafitteStéphaneCottarre-LafitteMarianneRéantPatriciaRoudautRaymondCe cours vidéo est tiré du cédérom Échocardiographie Doppler (éd. 2011), comportant plus de trente leçons (Écho-doppler transthoracique, Fonction systolique, Valvulopathies, Myocardiopathies,