Notice
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 : Impact of major clinical trials on ESC Chronic Heart Failure 2012 guidelines. Game changer trials: EMPHASIS-HF, SHIFT, Devices…
- document 1 document 2 document 3
- niveau 1 niveau 2 niveau 3
Descriptif
Title : Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 : Impact of major clinical trials on ESC Chronic Heart Failure 2012 guidelines. Game changer trials: EMPHASIS-HF, SHIFT, Devices…
Speaker: Faiez ZANNAD, Nancy, FR
Discussant: John CLELAND, Hull, GBR
Abstract : Impact of major clinical trials on ESC Chronic Heart Failure 2012 guidelines. Game changer trials: EMPHASIS-HF, SHIFT, Devices…
L’auteur n’a pas transmis de conflit d’intérêt concernant les données diffusées dans cette vidéo ou publiées dans la référence citée.
9th Global Cardiovascular Clinical Trialists Forum • Paris 2012
Multidisciplinary expert workshop : achievements challenges and barriers to implantation of the ESC 2012 chronic heart failure guidelines.
Chairpersons: Alain COHEN-SOLAL, Paris, FRA - Adrian VOORS, Groningen, NED
Background: The ESC-HFA chronic and acute heart failure guidelines have recently been published. However, the challenge for guidelines does not cease with a consensus document. Practical implementation is the critical step in establishing higher standards of care for individual patients. Improved guideline uptake is not only an index of better standards but a validation of the process of guideline production.
Improving consensus between guidelines is also important, differences in recommendations may act as a barrier to guideline. The NICE CHF guidance was updated in 2010, and it is not likely to be revised in short term.
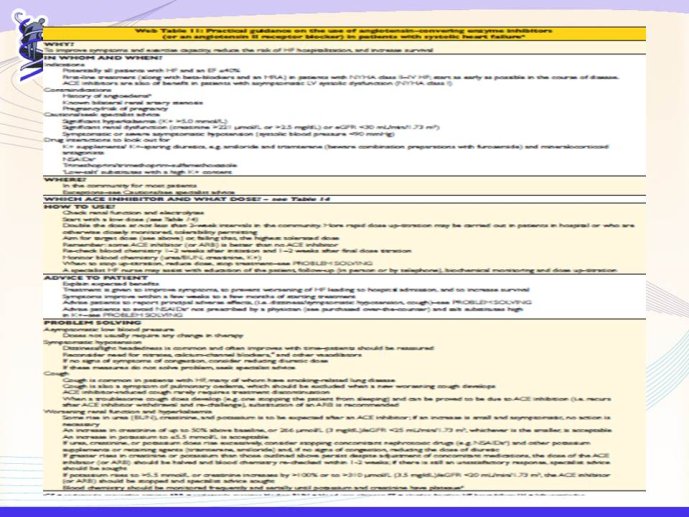
Practice differs from the guideline recommendations. Registries suggest differences in guideline interpretation and treatment/management of CHF between different stakeholders. Similarities and differences exist between GPs and hospital physicians’ approaches to management of CHF. One important issue that is not covered by the current guidelines is the class effect issue. Canadian and Australian CHF guideline and 2010 NICE guideline name eplerenone as preferred drug in heart failure, ESC mentions only mineralocorticoid receptor antagonists (MRAs.) as a class.
How to interpret compound vs. class effects while following guideline recommendations is an important issue.
Cost-effectiveness is a key not only to the content of guidelines but also in the assessment of implementation. Limits on healthcare resources mandate that resource-allocation decisions be guided by considerations of cost in relation to expected benefits. In cost-effectiveness analysis, the ratio of net healthcare costs to net health benefits provides an index by which priorities may be set.
Aims: This multidisciplinary consensus workshop aims at discussing CHF guideline implementation issues and the consequences on defining the place of MRA/eplerenone in management of CHF.
Réalisation, production : Canal U/3S et CERIMES
Keyword : Cardiovascular Clinical Trialists, Paris, 2012, Cardiovascular prevention, ESC-HFA, eplerenone
Dans la même collection
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
DeliargyrisEfthymiosMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Industry perspectiv…
WoehrleHolgerMODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
PrasadKrishnaMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : Different doses, differe…
VerheugtFreekMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Non randomized and/…
PocockStuart J.MODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
ZannadFaiezMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : How to secure the optima…
GibsonMichaelMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : Industry viewpoint (Joer…
KoeckJean-LouisMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 2 : Well Established Methods…
KoenigWolfgangMODIGLIANI Workshop 2 - Friday November 30, 2012 : ATHEROSCLEROSIS IMAGING IN CLINICAL TRIALS Facilitating the discovery of effective therapies Chairpersons: Jagat NARULA, New York, USA - Ahmed
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Lunch Session 1 : Options of and alte…
AbrahamWilliam T.MODIGLIANI Lunch Debate Session 1 - Friday November 30, 2012 THE DEVICE THERAPY TRIALISTS WORKSHOP Chairpersons: Gaetano DE FERRARI, Pavia, ITA - Ileana PIÑA, New York, USA Webcast: Tariq AHMAD,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : How to secure the optima…
GellerNancy L.MODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Debate Session 5 : Ultrafiltration fo…
RossiGian PaoloMODIGLIANI Debate Session 5 - Saturday December 1st, 2012 NOVEL DIURETIC STRATEGIES IN HEART FAILURE Chairpersons: Keld KJELDSEN, Copenhagen, DEN - Gian Paolo ROSSI, Padua, ITA Webcast: Patrick
Avec les mêmes intervenants et intervenantes
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 - Workshop 1 : New indications: Is hear…
ZannadFaiezMODIGLIANI Workshop 1 - Friday November 30, 2012 : THE THROMBOSIS TRIALISTS WORKSHOP DOSE AND TARGET PATIENT POPULATIONS ISSUES Chairpersons: Peter CLEMMENSEN, Copenhagen, DEN - George-Andrei DAN,
-
Semaine Médicale de Lorraine Nancy 2012 – Hypertension artérielle. Quelle prise en charge ?
ZannadFaiezT0403-06 Titre : Semaine Médicale de Lorraine Nancy 2012 – Hypertension artérielle. Quelle prise en charge ? Intervenant (e)(s) : Faiez ZANNAD (service de Cardiologie – CHU Nancy Brabois) Résumé :
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 : Omics research and system biology. Ke…
ZannadFaiezHow future trials may help optimizing benefit-to-risk ratio. The role of specialist scientific organizations. ISCP: Speaker: Felipe MARTINEZ, Cordoba, ARG CVCT: Speaker: Faiez ZANNAD, Nancy, FRA ESC
-
Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 : Autonomic modulation therapy for hear…
ZannadFaiezTitle : Cardiovascular Clinical Trialists (CVCT) Forum – Paris 2012 : Autonomic modulation therapy for heart failure: Preclinical data and ongoing trials Speaker: Faiez ZANNAD, Nancy, FRA Discussant:
-
Forum Sante : Vers un coeur immortel
KhalifeKhaliféZannadFaiezForum Santé : Vers un coeur immortel ?
-
Semaine de la Recherche Thérapeutique 26-03-09 Faïez ZANNAD
ZannadFaiezSemaine de la Recherche Thérapeutique, Faëz ZANNAD, Diabète, Cholestérol, Obésité
-
Semaine de la recherche thérapeutique - Faïez ZANNAD
ZannadFaiezConférence de Faïez ZANNAD pour le Débat Décideur
-
10ème colloque de biologie prospective-Epaisseur Intima-Média
ZannadFaiezLe complexe entre l'intima et la média est l'endroit ou se développe l'artéro-sclérose . L'épaisseur intima-media se produit plus tardivement chez les femmes que chez les hommes . Les facteurs de